Page 187 - Read Online
P. 187
Page 6 of 11 Hirschberg et al. Neuroimmunol Neuroinflammation 2018;5:27 I http://dx.doi.org/10.20517/2347-8659.2018.31
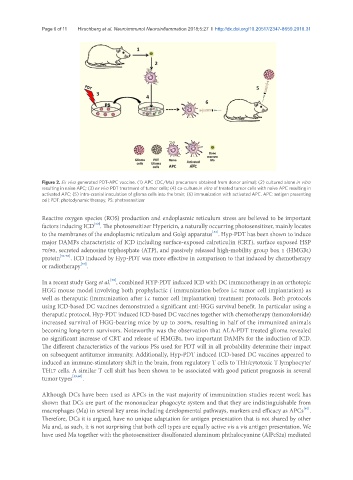
Figure 2. Ex vivo generated PDT-APC vaccine. (1) APC (DC/Ma) precursors obtained from donor animal; (2) cultured alone in vitro
resulting in naïve APC; (3) ex vivo PDT treatment of tumor cells; (4) co-culture in vitro of treated tumor cells with naïve APC resulting in
activated APC; (5) intra-cranial inoculation of glioma cells into the brain; (6) immunization with activated APC. APC: antigen presenting
cell; PDT: photodynamic therapy; PS: photosensitizer
Reactive oxygen species (ROS) production and endoplasmic reticulum stress are believed to be important
[52]
factors inducing ICD . The photosensitizer Hypericin, a naturally occurring photosensitizer, mainly locates
[53]
to the membranes of the endoplasmic reticulum and Golgi apparatus . Hyp-PDT has been shown to induce
major DAMPs characteristic of ICD including surface-exposed calreticulin (CRT), surface exposed HSP
70/90, secreted adenosine triphosphate (ATP), and passively released high-mobility group box 1 (HMGB1)
protein [54-56] . ICD induced by Hyp-PDT was more effective in comparison to that induced by chemotherapy
[57]
or radiotherapy .
[58]
In a recent study Garg et al. , combined HYP-PDT induced ICD with DC immunotherapy in an orthotopic
HGG mouse model involving both prophylactic ( immunization before i.c tumor cell implantation) as
well as theraputic (immunization after i.c tumor cell implantation) treatment protocols. Both protocols
using ICD-based DC vaccines demonstrated a significant anti-HGG survival benefit. In particular using a
theraputic protocol, Hyp-PDT induced ICD-based DC vaccines together with chemotherapy (temozolomide)
increased survival of HGG-bearing mice by up to 300%, resulting in half of the immunized animals
becoming long-term survivors. Noteworthy was the observation that ALA-PDT treated glioma revealed
no significant increase of CRT and release of HMGB1, two important DAMPs for the induction of ICD.
The different characteristics of the various PSs used for PDT will in all probability determine their impact
on subsequent antitumor immunity. Additionally, Hyp-PDT induced ICD-based DC vaccines appeared to
induced an immune-stimulatory shift in the brain, from regulatory T cells to TH1/cytotoxic T lymphocyte/
TH17 cells. A similar T cell shift has been shown to be associated with good patient prognosis in several
tumor types [59,60] .
Although DCs have been used as APCs in the vast majority of immunization studies recent work has
shown that DCs are part of the mononuclear phagocyte system and that they are indistinguishable from
macrophages (Ma) in several key areas including developmental pathways, markers and efficacy as APCs .
[61]
Therefore, DCs it is argued, have no unique adaptation for antigen presentation that is not shared by other
Ma and, as such, it is not surprising that both cell types are equally active vis a vis antigen presentation. We
have used Ma together with the photosensitizer disulfonated aluminum phthalocyanine (AlPcS2a) mediated