Page 32 - Read Online
P. 32
Min et al. Metab Target Organ Damage 2023;3:6 https://dx.doi.org/10.20517/mtod.2023.09 Page 3 of 10
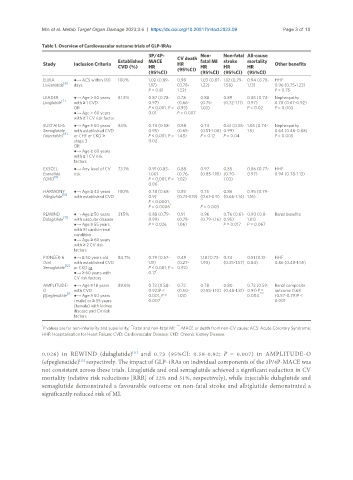
Table 1. Overview of Cardiovascular outcome trials of GLP-1RAs
3P/4P- CV death Non- Non-fatal All-cause
Study Inclusion Criteria Established MACE HR fatal MI stroke mortality Other benefits
HR
HR
CVD (%)
HR
HR
(95%CI) (95%CI) (95%CI) (95%CI) (95%CI)
ELIXA ●→ ACS within 180 100% 1.02 (0.89- 0.98 1.03 (0.87- 1.12 (0.79- 0.94 (0.78- HHF
Lixisenatide [6] days 1.17) (0.78- 1.22) 1.58) 1.13) 0.96 (0.75-1.23)
P = 0.81 1.22) P = 0.75
LEADER ●→ Age > 50 years 81.3% 0.87 (0.78- 0.78 0.88 0.89 0.85 (0.74- Nephropathy
[7]
Liraglutide with ≥ 1 CVD 0.97) (0.66- (0.75- (0.72-1.11) 0.97) 0.78 (0.67-0.92)
OR P < 0.001, P = 0.93) 1.03) P = 0.02 P = 0.003
*
●→ Age > 60 years 0.01 P = 0.007
with ≥ 1 CV risk factor
SUSTAIN-6 ●→ Age ≥ 50 years 83% 0.74 (0.58- 0.98 0.74 0.61 (0.38- 1.05 (0.74- Nephropathy
Semaglutide with established CVD 0.95) (0.65- (0.51-1.08) 0.99) 1.5) 0.64 (0.46-0.88)
(injectable) [8] or CHF or CKD ≥ P < 0.001, P = 1.48) P = 0.12 P = 0.04 P = 0.005
*
stage 3 0.02
OR
●→ Age ≥ 60 years
with ≥ 1 CV risk
factors
EXSCEL ●→ Any level of CV 73.1% 0.91 (0.83- 0.88 0.97 0.85 0.86 (0.77- HHF
Exenatide risk 1.00) (0.76- (0.85-1.10) (0.70- 0.97) 0.94 (0.78-1.13)
[9]
(QW) P < 0.001, P = 1.02) 1.03)
*
0.06
HARMONY ●→ Age ≥ 40 years 100% 0.78 (0.68- 0.93 0.75 0.86 0.95 (0.79-
[10]
Albiglutide with established CVD 0.9) (0.73-1.19) (0.61-0.9) (0.66-1.14) 1.16)
P < 0.0001, **
*
P = 0.0006 P = 0.003
REWIND ●→ Age ≥ 50 years 31.5% 0.88 (0.79- 0.91 0.96 0.76 (0.61- 0.90 (0.8- Renal benefits
[11]
Dulaglutide with vascular disease 0.99) (0.78- (0.79-1.16) 0.95) 1.01)
●→ Age ≥ 55 years P = 0.026 1.06) P = 0.017 P = 0.067
with ≥1 cardio-renal
condition
●→ Age ≥ 60 years
with ≥ 2 CV risk
factors
PIONEER-6 ●→ ≥ 50 years old 84.7% 0.79 (0.57- 0.49 1.18 (0.73- 0.74 0.51 (0.31- HHF
Oral with established CVD 1.11) (0.27- 1.90) (0.35-1.57) 0.84) 0.86 (0.48-1.55)
Semaglutide [12] or CKD or P < 0.001, P = 0.92)
*
●→ ≥ 60 years with 0.17
CV risk factors
AMPLITUDE- ●→ Age ≥ 18 years 89.6% 0.73 (0.58- 0.72 0.78 0.80 0.73 (0.59- Renal composite
O with CVD 0.92)P < (0.50- (0.55-1.10) (0.48-1.31) 0.91) P = outcome 0.68
Efpeglenatide [13 ●→ Age ≥ 50 years 0.001, P = 1.03) 0.004 *** (0.57-0.79)P <
]
(male) or ≥ 55 years 0.007 * 0.001
(female) with kidney
disease and CV risk
factors
* ** ***
P values are for non-inferiority and superiority; Fatal and non-fatal MI; MACE or death from non-CV cause; ACS: Acute Coronary Syndrome;
HHF: Hospitalisation for Heart Failure; CVD: Cardiovascular Disease; CKD: Chronic Kidney Disease.
0.026) in REWIND (dulaglutide) and 0.73 (95%CI: 0.58-0.92; P = 0.007) in AMPLITUDE-O
[11]
[13]
(efpeglenatide) respectively. The impact of GLP-1RAs on individual components of the 3P/4P-MACE was
not consistent across these trials. Liraglutide and oral semaglutide achieved a significant reduction in CV
mortality (relative risk reductions [RRR] of 22% and 51%, respectively), while injectable dulaglutide and
semaglutide demonstrated a favourable outcome on non-fatal stroke and albiglutide demonstrated a
significantly reduced risk of MI.