Page 31 - Read Online
P. 31
Llamoza-Torres et al. Metab Target Organ Damage 2024;4:40 https://dx.doi.org/10.20517/mtod.2024.64 Page 11 of 18
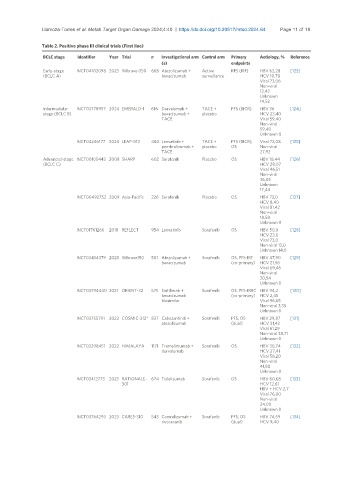
Table 2. Positive phase III clinical trials (First line)
BCLC stage Identifier Year Trial n Investigational arm Control arm Primary Aetiology, % Reference
(s) endpoints
Early-stage NCT04102098 2023 IMbrave 050 668 Atezolizumab + Active RFS (IRF) HBV 62,28 [123]
(BCLC A) bevacizumab surveillance HCV 10,78
Viral 73,06
Non-viral
12,42
Unknown
14,52
Intermediate- NCT03778957 2024 EMERALD-1 616 Durvalumab + TACE + PFS (BICR) HBV 36 [124]
stage (BCLC B) bevacizumab + placebo HCV 23,40
TACE Viral 59,40
Non-viral
59,40
Unknown 0
NCT04246177 2024 LEAP-012 480 Lenvatinib + TACE + PFS (BICR), Viral 72,08 [125]
pembrolizumab + placebo OS Non-viral
TACE 27,92
Advanced-stage NCT00105443 2008 SHARP 602 Sorafenib Placebo OS HBV 18,44 [126]
(BCLC C) HCV 28,07
Viral 46,51
Non-viral
36,05
Unknown
17,44
NCT00492752 2009 Asia-Pacific 226 Sorafenib Placebo OS HBV 73,0 [127]
HCV 8,40
Viral 81,42
Non-viral
18,58
Unknown 0
NCT01761266 2018 REFLECT 954 Lenvatinib Sorafenib OS HBV 50,0 [128]
HCV 23,0
Viral 73,0
Non-viral 13,0
Unknown 14,0
NCT03434379 2020 IMbrave150 501 Atezolizumab + Sorafenib OS, PFS-IRF HBV 47,90 [129]
bevacizumab (co-primary) HCV 21,56
Viral 69,46
Non-viral
30,54
Unknown 0
NCT03794440 2021 ORIENT-32 571 Sintilimab + Sorafenib OS, PFS-IRRC HBV 94,2 [130]
bevacizumab (co-primary) HCV 2,45
biosimilar Viral 96,65
Non-viral 3,35
Unknown 0
NCT03755791 2022 COSMIC-312* 837 Cabozantinib + Sorafenib PFS, OS HBV 29,87 [131]
atezolizumab (dual) HCV 31,42
Viral 61,29
Non-viral 38,71
Unknown 0
NCT03298451 2022 HIMALAYA 1171 Tremelimumab + Sorafenib OS HBV 30,74 [132]
durvalumab HCV 27,41
Viral 58,20
Non-viral
41,80
Unknown 0
NCT03412773 2023 RATIONALE- 674 Tislelizumab Sorafenib OS HBV 60,68 [133]
301 HCV 12,61
HBV + HCV 2,7
Viral 76,00
Non-viral
24,00
Unknown 0
NCT03764293 2023 CARES-310 543 Camrelizumab + Sorafenib PFS, OS HBV 74,59 [134]
rivoceranib (dual) HCV 9,40