Page 131 - Read Online
P. 131
Author Instructions
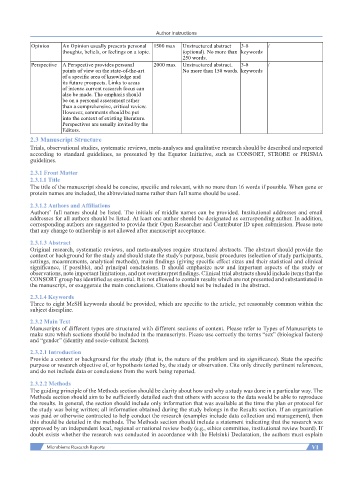
Opinion An Opinion usually presents personal 1500 max Unstructured abstract 3-8 /
thoughts, beliefs, or feelings on a topic. (optional). No more than keywords
250 words.
Perspective A Perspective provides personal 2000 max Unstructured abstract. 3-8 /
points of view on the state-of-the-art No more than 150 words. keywords
of a specific area of knowledge and
its future prospects. Links to areas
of intense current research focus can
also be made. The emphasis should
be on a personal assessment rather
than a comprehensive, critical review.
However, comments should be put
into the context of existing literature.
Perspectives are usually invited by the
Editors.
2.3 Manuscript Structure
Trials, observational studies, systematic reviews, meta-analyses and qualitative research should be described and reported
according to standard guidelines, as presented by the Equator Initiative, such as CONSORT, STROBE or PRISMA
guidelines.
2.3.1 Front Matter
2.3.1.1 Title
The title of the manuscript should be concise, specific and relevant, with no more than 16 words if possible. When gene or
protein names are included, the abbreviated name rather than full name should be used.
2.3.1.2 Authors and Affiliations
Authors’ full names should be listed. The initials of middle names can be provided. Institutional addresses and email
addresses for all authors should be listed. At least one author should be designated as corresponding author. In addition,
corresponding authors are suggested to provide their Open Researcher and Contributor ID upon submission. Please note
that any change to authorship is not allowed after manuscript acceptance.
2.3.1.3 Abstract
Original research, systematic reviews, and meta-analyses require structured abstracts. The abstract should provide the
context or background for the study and should state the study’s purpose, basic procedures (selection of study participants,
settings, measurements, analytical methods), main findings (giving specific effect sizes and their statistical and clinical
significance, if possible), and principal conclusions. It should emphasize new and important aspects of the study or
observations, note important limitations, and not overinterpret findings. Clinical trial abstracts should include items that the
CONSORT group has identified as essential. It is not allowed to contain results which are not presented and substantiated in
the manuscript, or exaggerate the main conclusions. Citations should not be included in the abstract.
2.3.1.4 Keywords
Three to eight MeSH keywords should be provided, which are specific to the article, yet reasonably common within the
subject discipline.
2.3.2 Main Text
Manuscripts of different types are structured with different sections of content. Please refer to Types of Manuscripts to
make sure which sections should be included in the manuscripts. Please use correctly the terms “sex” (biological factors)
and “gender” (identity and socio-cultural factors).
2.3.2.1 Introduction
Provide a context or background for the study (that is, the nature of the problem and its significance). State the specific
purpose or research objective of, or hypothesis tested by, the study or observation. Cite only directly pertinent references,
and do not include data or conclusions from the work being reported.
2.3.2.2 Methods
The guiding principle of the Methods section should be clarity about how and why a study was done in a particular way. The
Methods section should aim to be sufficiently detailed such that others with access to the data would be able to reproduce
the results. In general, the section should include only information that was available at the time the plan or protocol for
the study was being written; all information obtained during the study belongs in the Results section. If an organization
was paid or otherwise contracted to help conduct the research (examples include data collection and management), then
this should be detailed in the methods. The Methods section should include a statement indicating that the research was
approved by an independent local, regional or national review body (e.g., ethics committee, institutional review board). If
doubt exists whether the research was conducted in accordance with the Helsinki Declaration, the authors must explain
Microbiome Research Reports VI