Page 56 - Read Online
P. 56
Vojdeman et al. J Transl Genet Genom 2021;5:182-88 https://dx.doi.org/10.20517/jtgg.2021.03 Page 186
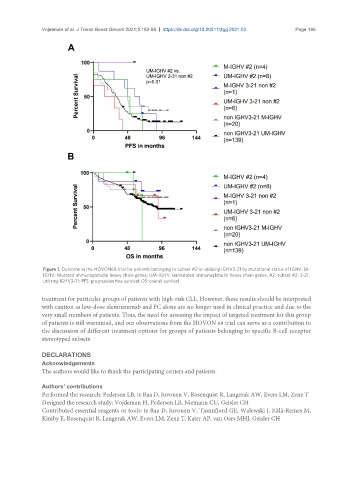
Figure 1. Outcome in the HOVON68 trial for patients belonging to subset #2 or utilizing IGHV3-21 by mutational status of IGHV. M-
IGHV: Mutated immunoglobulin heavy chain genes; UM-IGHV: unmutated immunoglobulin heavy chain genes; #2: subset #2; 3-21:
utilizing IGHV3-21; PFS: progression free survival; OS: overall survival.
treatment for particular groups of patients with high-risk CLL. However, these results should be interpreted
with caution as low-dose alemtuzumab and FC alone are no longer used in clinical practice and due to the
very small numbers of patients. Thus, the need for assessing the impact of targeted treatment for this group
of patients is still warranted, and our observations from the HOVON 68 trial can serve as a contribution to
the discussion of different treatment options for groups of patients belonging to specific B-cell receptor
stereotyped subsets.
DECLARATIONS
Acknowledgements
The authors would like to thank the participating centers and patients.
Authors’ contributions
Performed the research: Pedersen LB, te Raa D, Juvonen V, Rosenquist R, Langerak AW, Evers LM, Zenz T
Designed the research study: Vojdeman FJ, Pedersen LB, Niemann CU, Geisler CH
Contributed essential reagents or tools: te Raa D, Juvonen V, Tjønnfjord GE, Walewski J, Itälä-Remes M,
Kimby E, Rosenquist R, Langerak AW, Evers LM, Zenz T, Kater AP, van Oers MHJ, Geisler CH