Page 55 - Read Online
P. 55
Page 185 Vojdeman et al. J Transl Genet Genom 2021;5:182-88 https://dx.doi.org/10.20517/jtgg.2021.03
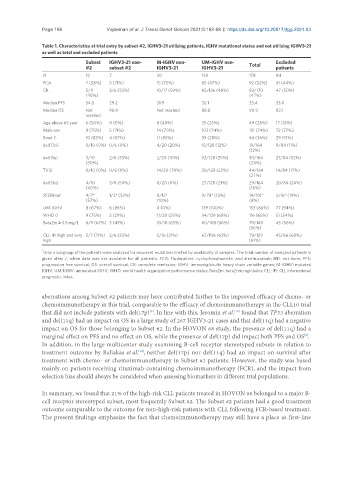
Table 1. Characteristics at trial entry by subset #2, IGHV3-21 utilizing patients, IGHV mutational status and not utilizing IGHV3-21
as well as total and excluded patients
Subset IGHV3-21 non- M-IGHV non- UM-IGHV non- Total Excluded
#2 subset #2 IGHV3-21 IGHV3-21 patients
N 12 7 20 139 178 94
FCA 7 (58%) 5 (71%) 15 (75%) 65 (47%) 92 (52%) 41 (44%)
CR 5/11 2/6 (33%) 10/17 (59%) 65/136 (48%) 83/170 47 (50%)
(45%) (47%)
Median PFS 54.5 29.2 31.9 32.1 33.4 33.0
Median OS Not 90.9 Not reached 88.8 90.9 82.1
reached
Age above 65 year 6 (50%) 0 (0%) 8 (40%) 35 (25%) 49 (28%) 17 (18%)
Male sex 9 (75%) 5 (71%) 14 (70%) 103 (74%) 131 (74%) 72 (77%)
Binet C 10 (83%) 4 (57%) 11 (55%) 39 (28%) 64 (36%) 29 (31%)
del(17p) 0/10 (0%) 0/6 (0%) 4/20 (20%) 15/128 (12%) 19/164 9/84 (11%)
(12%)
del(11q) 3/10 2/6 (33%) 2/20 (10%) 32/128 (25%) 39/164 27/84 (32%)
(30%) (24%)
Tri12 0/10 (0%) 0/6 (0%) 14/20 (70%) 30/128 (23%) 44/164 14/84 (17%)
(27%)
del(13q) 4/10 3/6 (50%) 0/20 (0%) 27/128 (21%) 29/164 20/84 (24%)
(40%) (18%)
SF3B1mut 4/7* 1/3* (33%) 0/13* 9/78* (12%) 14/101* 3/16* (19%)
(57%) (10%) (8%)
UM-IGHV 8 (67%) 6 (86%) 0 (0%) 139 (100%) 153 (86%) 77 (94%)
WHO 0 9 (75%) 2 (29%) 11/20 (55%) 94/139 (68%) 116 (65%) 51 (54%)
Beta2m ≥ 3.5 mg/L 6/9 (67%) 3 (43%) 10/16 (63%) 60/108 (56%) 79/140 45 (56%)
(56%)
CLL-IPI high and very 5/7 (71%) 2/6 (33%) 5/16 (31%) 67/106 (63%) 79/129 45/66 (68%)
high (61%)
*
Only a subgroup of the patients were analyzed for recurrent mutations limited by availability of samples. The total number of analyzed patients is
given after /, when data was not available for all patients. FCA: Fludarabine, cyclophosphamide, and alemtuzumab; ND: not done; PFS:
progression free survival; OS: overall survival; CR: complete remission; IGHV: immunoglobulin heavy chain variable genes; M-IGHV: mutated
IGHV; UM-IGHV: unmutated IGHV; WHO: world health organization performance status; Beta2m: beta2microglobulin; CLL-IPI: CLL international
prognostic index.
aberrations among Subset #2 patients may have contributed further to the improved efficacy of chemo- or
chemoimmunotherapy in this trial, comparable to the efficacy of chemoimmunotherapy in the CLL10 trial
that did not include patients with del(17p) . In line with this, Jeromin et al. found that TP53 aberration
[10]
[9]
and del(11q) had an impact on OS in a large study of 267 IGHV3-21 cases and that del(11q) had a negative
impact on OS for those belonging to Subset #2. In the HOVON 68 study, the presence of del(11q) had a
marginal effect on PFS and no effect on OS, while the presence of del(17p) did impact both PFS and OS .
[2]
In addition, in the large multicenter study examining B-cell receptor stereotyped subsets in relation to
[14]
treatment outcome by Baliakas et al. , neither del(17p) nor del(11q) had an impact on survival after
treatment with chemo- or chemoimmunotherapy in Subset #2 patients. However, the study was based
mainly on patients receiving rituximab-containing chemoimmunotherapy (FCR), and the impact from
selection bias should always be considered when assessing biomarkers in different trial populations.
In summary, we found that 21% of the high-risk CLL patients treated in HOVON 68 belonged to a major B-
cell receptor stereotyped subset, most frequently Subset #2. The Subset #2 patients had a good treatment
outcome comparable to the outcome for non-high-risk patients with CLL following FCR-based treatment.
The present findings emphasize the fact that chemoimmunotherapy may still have a place as first-line