Page 158 - Read Online
P. 158
Sadaf et al. J Transl Genet Genom 2022;6:63-83 https://dx.doi.org/10.20517/jtgg.2021.36 Page 77
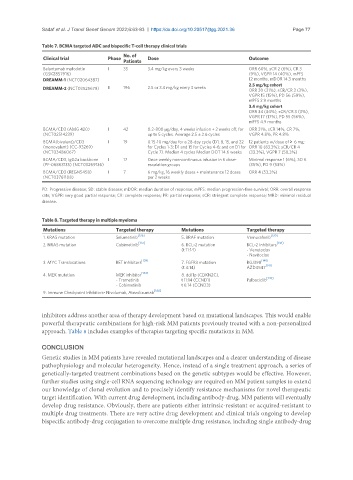
Table 7. BCMA targeted ADC and bispecific T-cell therapy clinical trials
No. of
Clinical trial Phase Dose Outcome
Patients
Belantamab mafodotin I 35 3.4 mg/kg every 3 weeks ORR 60%, sCR 2 (6%), CR 3
(GSK2857916) (9%), VGPR 14 (40%), mPFS
DREAMM-1 (NCT02064387) 12 months, mDOR 14.3 months
2.5 mg/kg cohort
DREAMM-2 (NCT03525678) II 196 2.5 or 3.4 mg/kg every 3 weeks
ORR 30 (31%), sCR/CR 3 (3%),
VGPR 15 (15%), PD 56 (58%),
mPFS 2.9 months
3.4 mg/kg cohort
ORR 34 (34%), sCR/CR 3 (3%),
VGPR 17 (17%), PD 55 (56%),
mPFS 4.9 months
BCMA/CD3 (AMG 420) I 42 0.2-800 μg/day, 4 weeks infusion + 2 weeks off, for ORR 31%, sCR 14%, CR 7%,
(NCT02514239) up to 5 cycles. Average 2.5 ± 2.6 cycles VGPR 4.8%, PR 4.8%
BCMA(bivalent)/CD3 I 19 0.15-10 mg/day for a 28-day cycle (D1, 8, 15, and 22 12 patients w/dose of ≥ 6 mg;
(monovalent) (CC-93269) for Cycles 1-3; D1 and 15 for Cycles 4-6; and on D1 for ORR 10 (83.3%); sCR/CR 4
(NCT03486067) Cycle 7). Median 4 cycles Median DOT 14.6 weeks (33.3%), VGPR 7 (58.3%)
BCMA/CD3, IgG2a backbone I 17 Once weekly non-continuous infusion in 6 dose- Minimal response 1 (6%), SD 6
(PF-06863135) (NCT03269136) escalation groups (35%), PD 9 (53%)
BCMA/CD3 (REGN5458) I 7 6 mg/kg, 16 weekly doses + maintenance 12 doses ORR 4 (53.3%)
(NCT03761108) per 2 weeks
PD: Progressive disease; SD: stable disease; mDOR: median duration of response; mPFS: median progression-free survival; ORR: overall response
rate; VGPR: very good partial response; CR: complete response; PR: partial response; sCR: stringent complete response; MRD: minimal residual
disease.
Table 8. Targeted therapy in multiple myeloma
Mutations Targeted therapy Mutations Targeted therapy
1. KRAS mutation Selumetinib [136] 5. BRAF mutation Vemurafenib [130]
[137] [138]
2. NRAS mutation Cobimetinib 6. BCL-2 mutation BCL-2 Inhibitors
(t 11:14) - Venetoclax
- Navitoclax
[139] [140]
3. MYC Translocations BET inhibitors 7. FGFR3 mutation BGJ398
(t 4:14) AZD4547 [141]
[142]
4. MEK mutation MEK inhibitor 8. del 1p (CDKN2C),
- Trametinib t 11:14 (CCND1) Palbociclib [143]
- Cobimetinib t 6:14 (CCND3)
9. Immune Checkpoint Inhibitors- Nivolumab, Atezolizumab [144]
inhibitors address another area of therapy development based on mutational landscapes. This would enable
powerful therapeutic combinations for high-risk MM patients previously treated with a non-personalized
approach. Table 8 includes examples of therapies targeting specific mutations in MM.
CONCLUSION
Genetic studies in MM patients have revealed mutational landscapes and a clearer understanding of disease
pathophysiology and molecular heterogeneity. Hence, instead of a single treatment approach, a series of
genetically-targeted treatment combinations based on the genetic subtypes would be effective. However,
further studies using single-cell RNA sequencing technology are required on MM patient samples to extend
our knowledge of clonal evolution and to precisely identify resistance mechanisms for novel therapeutic
target identification. With current drug development, including antibody-drug, MM patients will eventually
develop drug resistance. Obviously, there are patients either intrinsic-resistant or acquired-resistant to
multiple drug treatments. There are very active drug development and clinical trials ongoing to develop
bispecific antibody-drug conjugation to overcome multiple drug resistance, including single antibody-drug