Page 32 - Read Online
P. 32
Donskov et al. J Transl Genet Genom 2021;5:136-62 https://dx.doi.org/10.20517/jtgg.2021.12 Page 17
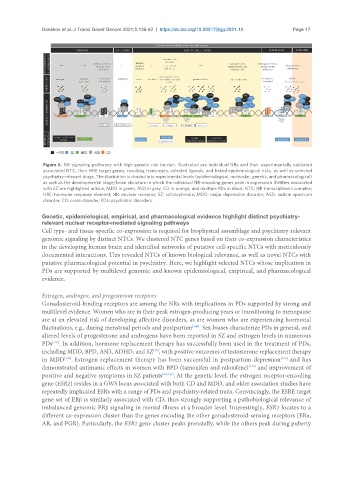
Figure 6. NR-signaling pathways with high genetic risk burden. Illustrated are individual NRs and their experimentally validated
associated NTC, their HRE target genes, resulting transcripts, selected ligands, and linked epidemiological risks, as well as selected
psychiatry-relevant drugs. The illustration is divided into experimental levels (epidemiological, molecular, genetic, and pharmacological)
as well as the developmental stage/brain structure in which the individual NR-encoding genes peak in expression. Entities associated
with SZ are highlighted in blue, MDD in green, ASD in grey, CD in orange, and multiple PDs in black. NTC: NR transcriptomic complex;
HRE: hormone response element; NR: nuclear receptor; SZ: schizophrenia; MDD: major depressive disorder; ASD: autism spectrum
disorder; CD: cross-disorder; PDs: psychiatric disorders.
Genetic, epidemiological, empirical, and pharmacological evidence highlight distinct psychiatry-
relevant nuclear receptor-mediated signaling pathways
Cell type- and tissue-specific co-expression is required for biophysical assemblage and psychiatry-relevant
genomic signaling by distinct NTCs. We clustered NTC genes based on their co-expression characteristics
in the developing human brain and identified networks of putative cell-specific NTCs with meticulously
documented interactions. This revealed NTCs of known biological relevance, as well as novel NTCs with
putative pharmacological potential in psychiatry. Here, we highlight selected NTCs whose implication in
PDs are supported by multilevel genomic and known epidemiological, empirical, and pharmacological
evidence.
Estrogen, androgen, and progesterone receptors
Gonadosteroid-binding receptors are among the NRs with implications in PDs supported by strong and
multilevel evidence. Women who are in their peak estrogen-producing years or transitioning to menopause
are at an elevated risk of developing affective disorders, as are women who are experiencing hormonal
fluctuations, e.g., during menstrual periods and postpartum . Sex-biases characterize PDs in general, and
[140]
altered levels of progesterone and androgens have been reported in SZ and estrogen levels in numerous
PDs . In addition, hormone replacement therapy has successfully been used in the treatment of PDs,
[141]
including MDD, BPD, ASD, ADHD, and SZ , with positive outcomes of testosterone replacement therapy
[35]
in MDD . Estrogen replacement therapy has been successful in postpartum depression and has
[142]
[142]
[143]
demonstrated antimanic effects in women with BPD (tamoxifen and raloxifene) and improvement of
positive and negative symptoms in SZ patients [35,144] . At the genetic level, the estrogen receptor-encoding
gene (ESR2) resides in a GWS locus associated with both CD and MDD, and older association studies have
repeatedly implicated ESRs with a range of PDs and psychiatry-related traits. Convincingly, the ESRE target
gene set of ERβ is similarly associated with CD, thus strongly supporting a pathobiological relevance of
imbalanced genomic ERβ signaling in mental illness at a broader level. Interestingly, ESR2 locates to a
different co-expression cluster than the genes encoding the other gonadosteroid-sensing receptors (ERα,
AR, and PGR). Particularly, the ESR2 gene cluster peaks prenatally, while the others peak during puberty