Page 42 - Read Online
P. 42
Page 6 of 10 Boshe et al. J Transl Genet Genom 2018;2:12. I https://doi.org/10.20517/jtgg.2018.18
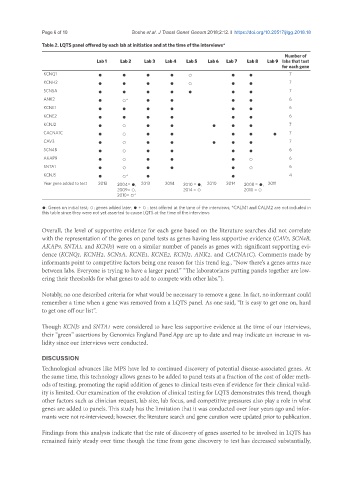
Table 2. LQTS panel offered by each lab at initiation and at the time of the interviews*
Number of
Lab 1 Lab 2 Lab 3 Lab 4 Lab 5 Lab 6 Lab 7 Lab 8 Lab 9 labs that test
for each gene
KCNQ1 ● ● ● ● ○ ● ● 7
KCNH2 ● ● ● ● ○ ● ● 7
SCN5A ● ● ● ● ● ● ● 7
ANK2 ● ○* ● ● ● ● 6
KCNE1 ● ● ● ● ● ● 6
KCNE2 ● ● ● ● ● ● 6
KCNJ2 ● ○ ● ● ● ● ● 7
CACNA1C ● ○ ● ● ● ● ● 7
CAV3 ● ○ ● ● ● ● ● 7
SCN4B ● ○ ● ● ● ● 6
AKAP9 ● ○ ● ● ● ○ 6
SNTA1 ● ○ ● ● ● ○ 6
KCNJ5 ● ○* ● ● 4
Year gene added to test 2013 2004= ●, 2013 2014 2010 = ●, 2010 2014 2008 = ●, 2011
2009= ○, 2014 = ○ 2010 = ○
2010= ○*
●: Genes on initial test; ○: genes added later; ● + ○ : test offered at the time of the interviews; *CALM1 and CALM2 are not included in
this table since they were not yet asserted to cause LQTS at the time of the interviews
Overall, the level of supportive evidence for each gene based on the literature searches did not correlate
with the representation of the genes on panel tests as genes having less supportive evidence (CAV3, SCN4B,
AKAP9, SNTA1, and KCNJ5) were on a similar number of panels as genes with significant supporting evi-
dence (KCNQ1, KCNH2, SCN5A, KCNE1, KCNE2, KCNJ2, ANK2, and CACNA1C). Comments made by
informants point to competitive factors being one reason for this trend (e.g., “Now there’s a genes arms race
between labs. Everyone is trying to have a larger panel.” “The laboratorians putting panels together are low-
ering their thresholds for what genes to add to compete with other labs.”).
Notably, no one described criteria for what would be necessary to remove a gene. In fact, no informant could
remember a time when a gene was removed from a LQTS panel. As one said, “It is easy to get one on, hard
to get one off our list”.
Though KCNJ5 and SNTA1 were considered to have less supportive evidence at the time of our interviews,
their “green” assertions by Genomics England PanelApp are up to date and may indicate an increase in va-
lidity since our interviews were conducted.
DISCUSSION
Technological advances like MPS have led to continued discovery of potential disease-associated genes. At
the same time, this technology allows genes to be added to panel tests at a fraction of the cost of older meth-
ods of testing, promoting the rapid addition of genes to clinical tests even if evidence for their clinical valid-
ity is limited. Our examination of the evolution of clinical testing for LQTS demonstrates this trend, though
other factors such as clinician request, lab size, lab focus, and competitive pressures also play a role in what
genes are added to panels. This study has the limitation that it was conducted over four years ago and infor-
mants were not re-interviewed; however, the literature search and gene curation were updated prior to publication.
Findings from this analysis indicate that the rate of discovery of genes asserted to be involved in LQTS has
remained fairly steady over time though the time from gene discovery to test has decreased substantially,