Page 20 - Read Online
P. 20
Page 4 of 9 Hong et al. J Transl Genet Genom 2018;2:8. I https://doi.org/10.20517/jtgg.2018.06
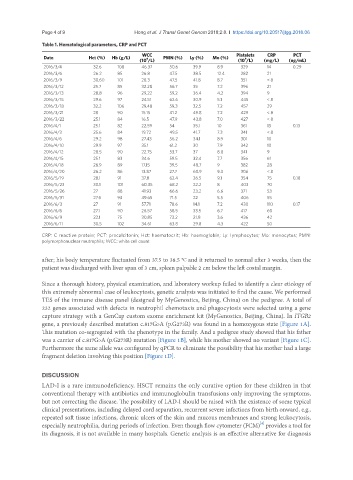
Table 1. Hematological parameters, CRP and PCT
WCC Platelets CRP PCT
Date Hct (%) Hb (g/L) 9 PMN (%) Ly (%) Mo (%) 9
(10 /L) (10 /L) (mg/L) (ng/mL)
2016/3/4 32.6 108 46.37 50.6 39.9 8.9 329 14 0.29
2016/3/6 26.2 85 26.8 47.5 38.5 12.4 282 21
2016/3/9 30.60 101 28.3 47.5 41.8 8.7 351 < 8
2016/3/12 25.7 85 32.28 56.7 35 7.2 396 21
2016/3/13 28.8 96 29.22 59.2 36.4 4.2 394 9
2016/3/15 29.6 97 24.51 62.6 30.9 5.1 445 < 8
2016/3/18 32.2 106 29.48 59.3 32.5 7.2 457 29
2016/3/21 28 90 15.15 41.2 49.8 7.2 429 < 8
2016/3/22 25.1 84 16.5 47.9 43.8 7.0 427 < 8
2016/4/1 25.1 82 22.59 54 35.1 10 361 13 0.13
2016/4/3 25.6 84 19.72 49.5 41.7 7.3 341 < 8
2016/4/6 29.2 98 27.43 56.2 34.1 8.9 301 10
2016/4/10 29.9 97 35.1 61.2 30 7.9 342 10
2016/4/12 28.5 90 22.75 53.7 37 8.8 341 9
2016/4/15 25.1 83 34.6 59.5 32.4 7.7 356 61
2016/4/18 26.9 89 17.15 39.5 48.7 9 382 28
2016/4/20 26.2 86 13.87 27.7 60.9 9.3 306 < 8
2016/5/19 28.1 91 37.8 62.4 26.5 9.1 354 75 0.18
2016/5/23 30.1 101 60.35 68.2 22.2 8 403 70
2016/5/26 27 88 41.93 66.6 23.2 6.6 371 53
2016/5/31 27.6 93 49.65 71.5 22 5.5 406 55
2016/6/3 27 91 57.71 78.6 14.1 7.2 430 110 0.17
2016/6/6 27.1 90 26.57 58.5 33.5 6.7 417 60
2016/6/9 22.1 75 30.85 73.2 21.8 3.6 436 42
2016/6/11 30.5 102 34.61 63.8 29.8 4.3 422 50
CRP: C reactive protein; PCT: procalcitonin; Hct: haematocrit; Hb: haemoglobin; Ly: lymphocytes; Mo: monocytes; PMN:
polymorphonuclear neutrophils; WCC: white cell count
after; his body temperature fluctuated from 37.5 to 38.5 °C and it returned to normal after 3 weeks, then the
patient was discharged with liver span of 3 cm, spleen palpable 2 cm below the left costal margin.
Since a thorough history, physical examination, and laboratory workup failed to identify a clear etiology of
this extremely abnormal case of leukocytosis, genetic analysis was initiated to find the cause. We performed
TES of the immune disease panel (designed by MyGenostics, Beijing, China) on the pedigree. A total of
232 genes associated with defects in neutrophil chemotaxis and phagocytosis were selected using a gene
capture strategy with a GenCap custom exome enrichment kit (MyGenostics, Beijing, China). In ITGB2
gene, a previously described mutation c.817G>A (p.G273R) was found in a homozygous state [Figure 1A].
This mutation co-segregated with the phenotype in the family. And a pedigree study showed that his father
was a carrier of c.817G>A (p.G273R) mutation [Figure 1B], while his mother showed no variant [Figure 1C].
Furthermore the same allele was configured by qPCR to eliminate the possibility that his mother had a large
fragment deletion involving this position [Figure 1D].
DISCUSSION
LAD-I is a rare immunodeficiency. HSCT remains the only curative option for these children in that
conventional therapy with antibiotics and immunoglobulin transfusions only improving the symptoms,
but not correcting the disease. The possibility of LAD-I should be raised with the existence of some typical
clinical presentations, including delayed cord separation, recurrent severe infections from birth onward, e.g.,
repeated soft tissue infections, chronic ulcers of the skin and mucous membranes and strong leukocytosis,
[4]
especially neutrophilia, during periods of infection. Even though flow cytometer (FCM) provides a tool for
its diagnosis, it is not available in many hospitals. Genetic analysis is an effective alternative for diagnosis