Page 74 - Read Online
P. 74
Thomas et al. J Transl Genet Genom 2024;8:249-77 https://dx.doi.org/10.20517/jtgg.2024.15 Page 251
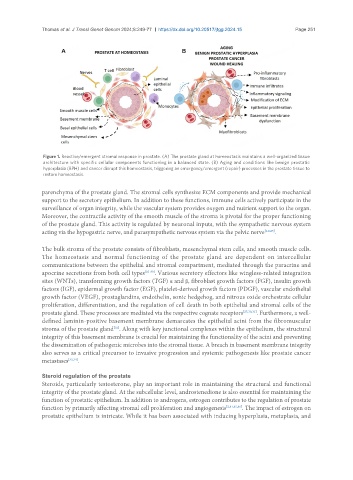
Figure 1. Reactive/emergent stromal response in prostate. (A) The prostate gland at homeostasis maintains a well-organized tissue
architecture with specific cellular components functioning in a balanced state. (B) Aging and conditions like benign prostatic
hyperplasia (BPH) and cancer disrupt this homeostasis, triggering an emergency/emergent (repair) processes in the prostate tissue to
restore homeostasis.
parenchyma of the prostate gland. The stromal cells synthesize ECM components and provide mechanical
support to the secretory epithelium. In addition to these functions, immune cells actively participate in the
surveillance of organ integrity, while the vascular system provides oxygen and nutrient support to the organ.
Moreover, the contractile activity of the smooth muscle of the stroma is pivotal for the proper functioning
of the prostate gland. This activity is regulated by neuronal inputs, with the sympathetic nervous system
acting via the hypogastric nerve, and parasympathetic nervous system via the pelvic nerve [1,2,25] .
The bulk stroma of the prostate consists of fibroblasts, mesenchymal stem cells, and smooth muscle cells.
The homeostasis and normal functioning of the prostate gland are dependent on intercellular
communications between the epithelial and stromal compartment, mediated through the paracrine and
apocrine secretions from both cell types [25-30] . Various secretory effectors like wingless-related integration
sites (WNTs), transforming growth factors (TGF) α and β, fibroblast growth factors (FGF), insulin growth
factors (IGF), epidermal growth factor (EGF), platelet-derived growth factors (PDGF), vascular endothelial
growth factor (VEGF), prostaglandins, endothelin, sonic hedgehog, and nitrous oxide orchestrate cellular
proliferation, differentiation, and the regulation of cell death in both epithelial and stromal cells of the
prostate gland. These processes are mediated via the respective cognate receptors [27,30,31] . Furthermore, a well-
defined laminin-positive basement membrane demarcates the epithelial acini from the fibromuscular
stroma of the prostate gland . Along with key junctional complexes within the epithelium, the structural
[32]
integrity of this basement membrane is crucial for maintaining the functionality of the acini and preventing
the dissemination of pathogenic microbes into the stromal tissue. A breach in basement membrane integrity
also serves as a critical precursor to invasive progression and systemic pathogenesis like prostate cancer
metastases [33,34] .
Steroid regulation of the prostate
Steroids, particularly testosterone, play an important role in maintaining the structural and functional
integrity of the prostate gland. At the subcellular level, androstenedione is also essential for maintaining the
function of prostatic epithelium. In addition to androgens, estrogen contributes to the regulation of prostate
function by primarily affecting stromal cell proliferation and angiogenesis [2,31,35,36] . The impact of estrogen on
prostatic epithelium is intricate. While it has been associated with inducing hyperplasia, metaplasia, and