Page 45 - Read Online
P. 45
Bibi et al. J Transl Genet Genom 2024;8:119-161 https://dx.doi.org/10.20517/jtgg.2023.50 Page 135
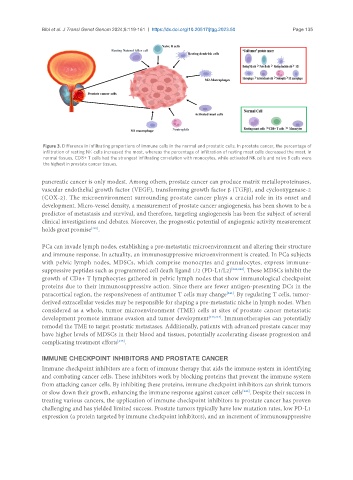
Figure 3. Difference in infiltrating proportions of immune cells in the normal and prostatic cells. In prostate cancer, the percentage of
infiltration of resting NK cells increased the most, whereas the percentage of infiltration of resting mast cells decreased the most. In
normal tissues, CD8+ T cells had the strongest infiltrating correlation with monocytes, while activated NK cells and naive B cells were
the highest in prostate cancer tissues.
pancreatic cancer is only modest. Among others, prostate cancer can produce matrix metalloproteinases,
vascular endothelial growth factor (VEGF), transforming growth factor β (TGFβ), and cyclooxygenase-2
(COX-2). The microenvironment surrounding prostate cancer plays a crucial role in its onset and
development. Micro-vessel density, a measurement of prostate cancer angiogenesis, has been shown to be a
predictor of metastasis and survival, and therefore, targeting angiogenesis has been the subject of several
clinical investigations and debates. Moreover, the prognostic potential of angiogenic activity measurement
[240]
holds great promise .
PCa can invade lymph nodes, establishing a pre-metastatic microenvironment and altering their structure
and immune response. In actuality, an immunosuppressive microenvironment is created. In PCa subjects
with pelvic lymph nodes, MDSCs, which comprise monocytes and granulocytes, express immune-
suppressive peptides such as programmed cell death ligand 1/2 (PD-L1/L2) [241,242] . These MDSCs inhibit the
growth of CD8+ T lymphocytes gathered in pelvic lymph nodes that show immunological checkpoint
proteins due to their immunosuppressive action. Since there are fewer antigen-presenting DCs in the
paracortical region, the responsiveness of antitumor T cells may change . By regulating T cells, tumor-
[241]
derived extracellular vesicles may be responsible for shaping a pre-metastatic niche in lymph nodes. When
considered as a whole, tumor microenvironment (TME) cells at sites of prostate cancer metastatic
development promote immune evasion and tumor development [243,244] . Immunotherapies can potentially
remodel the TME to target prostatic metastases. Additionally, patients with advanced prostate cancer may
have higher levels of MDSCs in their blood and tissues, potentially accelerating disease progression and
complicating treatment efforts .
[245]
IMMUNE CHECKPOINT INHIBITORS AND PROSTATE CANCER
Immune checkpoint inhibitors are a form of immune therapy that aids the immune system in identifying
and combating cancer cells. These inhibitors work by blocking proteins that prevent the immune system
from attacking cancer cells. By inhibiting these proteins, immune checkpoint inhibitors can shrink tumors
or slow down their growth, enhancing the immune response against cancer cells . Despite their success in
[246]
treating various cancers, the application of immune checkpoint inhibitors to prostate cancer has proven
challenging and has yielded limited success. Prostate tumors typically have low mutation rates, low PD-L1
expression (a protein targeted by immune checkpoint inhibitors), and an increment of immunosuppressive