Page 81 - Read Online
P. 81
Lorenzin et al. J Transl Genet Genom 2019;3:5. I https://doi.org/10.20517/jtgg.2019.01 Page 3 of 12
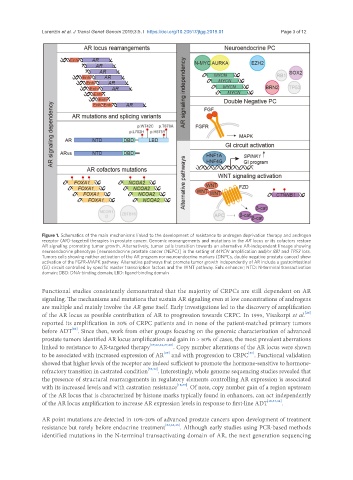
Figure 1. Schematics of the main mechanisms linked to the development of resistance to androgen deprivation therapy and androgen
receptor (AR)-targeted therapies in prostate cancer. Genomic rearrangements and mutations in the AR locus or its cofactors restore
AR signaling promoting tumor growth. Alternatively, tumor cells transition towards an alternative AR-independent lineage showing
neuroendocrine phenotype [neuroendocrine prostate cancer (NEPC)] in the setting of MYCN amplification and/or RB1 and TP53 loss.
Tumors cells showing neither activation of the AR program nor neuroendocrine markers (DNPCs, double negative prostate cancer) show
activation of the FGFR-MAPK pathway. Alternative pathways that promote tumor growth independently of AR include a gastrointestinal
(GI) circuit controlled by specific master transcription factors and the WNT pathway. Enh: enhancer; NTD: N-terminal transactivation
domain; DBD: DNA-binding domain; LBD: ligand-binding domain
Functional studies consistently demonstrated that the majority of CRPCs are still dependent on AR
signaling. The mechanisms and mutations that sustain AR signaling even at low concentrations of androgens
are multiple and mainly involve the AR gene itself. Early investigations led to the discovery of amplification
of the AR locus as possible contribution of AR to progression towards CRPC. In 1995, Visakorpi et al. [26]
reported its amplification in 30% of CRPC patients and in none of the patient-matched primary tumors
[26]
before ADT . Since then, work from other groups focusing on the genomic characterization of advanced
prostate tumors identified AR locus amplification and gain in > 50% of cases, the most prevalent aberrations
linked to resistance to AR-targeted therapy [19,22,24,27,28] . Copy number alterations of the AR locus were shown
[30]
[29]
to be associated with increased expression of AR and with progression to CRPC . Functional validation
showed that higher levels of the receptor are indeed sufficient to promote the hormone-sensitive to hormone-
refractory transition in castrated condition [31,32] . Interestingly, whole genome sequencing studies revealed that
the presence of structural rearrangements in regulatory elements controlling AR expression is associated
with its increased levels and with castration resistance [18,33] . Of note, copy number gain of a region upstream
of the AR locus that is characterized by histone marks typically found in enhancers, can act independently
of the AR locus amplification to increase AR expression levels in response to first-line ADT [18,33,34] .
AR point mutations are detected in 10%-20% of advanced prostate cancers upon development of treatment
resistance but rarely before endocrine treatment [23,35,36] . Although early studies using PCR-based methods
identified mutations in the N-terminal transactivating domain of AR, the next generation sequencing