Page 70 - Read Online
P. 70
Fraser. J Transl Genet Genom 2018;2:21. I https://doi.org/10.20517/jtgg.2018.27 Page 7 of 15
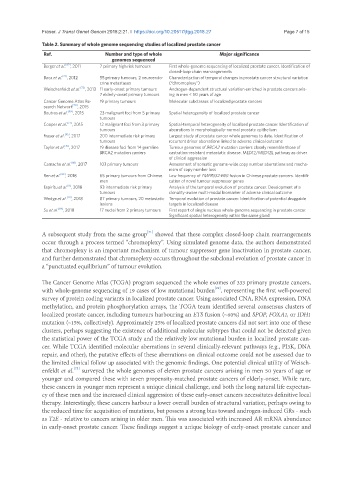
Table 2. Summary of whole genome sequencing studies of localized prostate cancer
Ref. Number and type of whole Major significance
genomes sequenced
Berger et al. [69] , 2011 7 primary high risk tumours First whole-genome sequencing of localized prostate cancer. Identification of
closed-loop chain rearrangements
Baca et al. , 2012 55 primary tumours, 2 neuroendo- Characterization of temporal changes in prostate cancer structural variation
[71]
crine metastases (“chromoplexy”)
Weischenfeldt et al. [72] , 2013 11 early-onset primary tumours Androgen-dependent structural variation enriched in prostate cancers aris-
7 elderly-onset primary tumours ing in men < 50 years of age
Cancer Genome Atlas Re- 19 primary tumours Molecular subclasses of localized prostate cancers
search Network [29] , 2015
Boutros et al. [49] , 2015 23 malignant foci from 5 primary Spatial heterogeneity of localized prostate cancer
tumours
Cooper et al. [77] , 2015 12 malignant foci from 3 primary Spatial-temporal heterogeneity of localized prostate cancer. Identification of
tumours aberrations in morphologically-normal prostate epithelium
Fraser et al. [10] , 2017 200 intermediate risk primary Largest study of prostate cancer whole genomes to date. Identification of
tumours recurrent driver aberrations linked to adverse clinical outcome
Taylor et al. , 2017 19 disease foci from 14 germline Tumour genomes of BRCA2 mutation carriers closely resemble those of
[15]
BRCA2 mutation carriers castration-resistant metastatic disease. MED12/MED12L pathway as driver
of clinical aggression
Camacho et al. [88] , 2017 103 primary tumours Assessment of somatic genome-wide copy number aberrations and mecha-
nism of copy number loss
Ren et al. [89] , 2018 65 primary tumours from Chinese Low frequency of TMPRSS2:ERG fusion in Chinese prostate cancers. Identifi-
men cation of novel tumour suppressor genes
Espiritu et al. , 2018 93 intermediate risk primary Analysis of the temporal evolution of prostate cancer. Development of a
[9]
tumours clonality-aware multi-modal biomarker of adverse clinical outcome
Wedge et al. [79] , 2018 87 primary tumours, 20 metastatic Temporal evolution of prostate cancer. Identification of potential druggable
lesions targets in localized disease
Su et al. [84] , 2018 17 nuclei from 2 primary tumours First report of single nucleus whole-genome sequencing in prostate cancer.
Significant spatial heterogeneity within the same gland
[71]
A subsequent study from the same group showed that these complex closed-loop chain rearrangements
occur through a process termed “chromoplexy”. Using simulated genome data, the authors demonstrated
that chromoplexy is an important mechanism of tumour suppressor gene inactivation in prostate cancer,
and further demonstrated that chromoplexy occurs throughout the subclonal evolution of prostate cancer in
a “punctuated equilibrium” of tumour evolution.
The Cancer Genome Atlas (TCGA) program sequenced the whole exomes of 333 primary prostate cancers,
[29]
with whole-genome sequencing of 19 cases of low mutational burden , representing the first well-powered
survey of protein coding variants in localized prostate cancer. Using associated CNA, RNA expression, DNA
methylation, and protein phosphorylation arrays, the TCGA team identified several consensus clusters of
localized prostate cancer, including tumours harbouring an ETS fusion (~60%) and SPOP, FOXA1, or IDH1
mutation (~15%, collectively). Approximately 25% of localized prostate cancers did not sort into one of these
clusters, perhaps suggesting the existence of additional molecular subtypes that could not be detected given
the statistical power of the TCGA study and the relatively low mutational burden in localized prostate can-
cer. While TCGA identified molecular aberrations in several clinically-relevant pathways (e.g., PI3K, DNA
repair, and other), the putative effects of these aberrations on clinical outcome could not be assessed due to
the limited clinical follow up associated with the genomic findings. One potential clinical utility of Weisch-
[72]
enfeldt et al. surveyed the whole genomes of eleven prostate cancers arising in men 50 years of age or
younger and compared these with seven propensity-matched prostate cancers of elderly-onset. While rare,
these cancers in younger men represent a unique clinical challenge, and both the long natural life expectan-
cy of these men and the increased clinical aggression of these early-onset cancers necessitates definitive local
therapy. Interestingly, these cancers harbour a lower overall burden of structural variation, perhaps owing to
the reduced time for acquisition of mutations, but possess a strong bias toward androgen-induced GRs - such
as T2E - relative to cancers arising in older men. This was associated with increased AR mRNA abundance
in early-onset prostate cancer. These findings suggest a unique biology of early-onset prostate cancer and