Page 105 - Read Online
P. 105
Keung et al. J Transl Genet Genom 2019;3:8. I https://doi.org/10.20517/jtgg.2019.03 Page 3 of 9
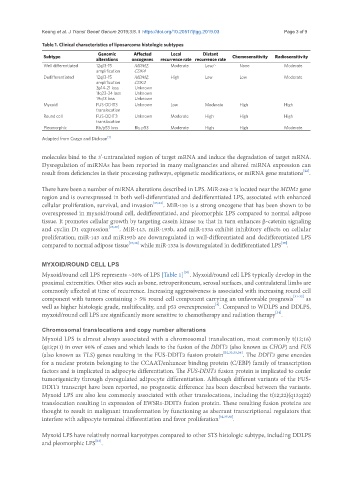
Table 1. Clinical characteristics of liposarcoma histologic subtypes
Subtype Genomic Affected Local Distant Chemosensitivity Radiosensitivity
alterations oncogenes recurrence rate recurrence rate
Well differentiated 12q13-15 MDM2, Moderate Low/- None Moderate
amplification CDK4
Dedifferentiated 12q13-15 MDM2, High Low Low Moderate
amplification CDK4
3p14-21 loss Unknown
11q23-24 loss Unknown
19q13 loss Unknown
Myxoid FUS-DDIT3 Unknown Low Moderate High High
translocation
Round cell FUS-DDIT3 Unknown Moderate High High High
translocation
Pleomorphic Rb/p53 loss Rb, p53 Moderate High High Moderate
Adapted from Crago and Dickson [1]
molecules bind to the 3’-untranslated region of target mRNA and induce the degradation of target mRNA.
Dysregulation of miRNAs has been reported in many malignancies and altered miRNA expression can
[22]
result from deficiencies in their processing pathways, epigenetic modifications, or miRNA gene mutations .
There have been a number of miRNA alterations described in LPS. MiR-26a-2 is located near the MDM2 gene
region and is overexpressed in both well-differentiated and dedifferentiated LPS, associated with enhanced
cellular proliferation, survival, and invasion [23,24] . MiR-155 is a strong oncogene that has been shown to be
overexpressed in myxoid/round cell, dedifferentiated, and pleomorphic LPS compared to normal adipose
tissue. It promotes cellular growth by targeting casein kinase 1α that in turn enhances β-catenin signaling
and cyclin D1 expression [25,26] . MiR-143, miR-193b, and miR-133a exhibit inhibitory effects on cellular
proliferation; miR-143 and miR193b are downregulated in well-differentiated and dedifferentiated LPS
[29]
compared to normal adipose tissue [27,28] while miR-133a is downregulated in dedifferentiated LPS .
MYXOID/ROUND CELL LPS
[30]
Myxoid/round cell LPS represents ~30% of LPS [Table 1] . Myxoid/round cell LPS typically develop in the
proximal extremities. Other sites such as bone, retroperitoneum, serosal surfaces, and contralateral limbs are
commonly affected at time of recurrence. Increasing aggressiveness is associated with increasing round cell
component with tumors containing > 5% round cell component carrying an unfavorable prognosis [31-33] as
[4]
well as higher histologic grade, multifocality, and p53 overexpression . Compared to WDLPS and DDLPS,
[34]
myxoid/round cell LPS are significantly more sensitive to chemotherapy and radiation therapy .
Chromosomal translocations and copy number alterations
Myxoid LPS is almost always associated with a chromosomal translocation, most commonly t(12;16)
(q13;p11) in over 90% of cases and which leads to the fusion of the DDIT3 (also known as CHOP) and FUS
(also known as TLS) genes resulting in the FUS-DDIT3 fusion protein [32,33,35,36] . The DDIT3 gene encodes
for a nuclear protein belonging to the CCAAT/enhancer binding protein (C/EBP) family of transcription
factors and is implicated in adipocyte differentiation. The FUS-DDIT3 fusion protein is implicated to confer
tumorigenicity through dysregulated adipocyte differentiation. Although different variants of the FUS-
DDIT3 transcript have been reported, no prognostic difference has been described between the variants.
Myxoid LPS are also less commonly associated with other translocations, including the t(12;22)(q13;q22)
translocation resulting in expression of EWSR1-DDIT3 fusion protein. These resulting fusion proteins are
thought to result in malignant transformation by functioning as aberrant transcriptional regulators that
interfere with adipocyte terminal differentiation and favor proliferation [32,37,38] .
Myxoid LPS have relatively normal karyotypes compared to other STS histologic subtype, including DDLPS
[12]
and pleomorphic LPS .