Page 19 - Read Online
P. 19
Lambert et al. J Transl Genet Genom 2018;2:11. I https://doi.org/10.20517/jtgg.2018.11 Page 5 of 10
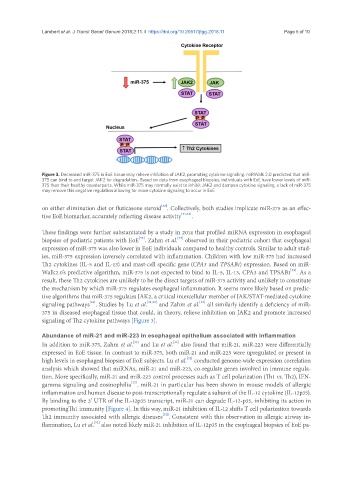
Figure 3. Decreased miR-375 in EoE tissue may relieve inhibition of JAK2, promoting cytokine signaling. miRWalk 2.0 predicted that miR-
375 can bind to and target JAK2 for degradation.. Based on data from esophageal biopsies, individuals with EoE have lower levels of miR-
375 than their healthy counterparts. While miR-375 may normally exist to inhibit JAK2 and dampen cytokine signaling, a lack of miR-375
may remove this negative regulation allowing for more cytokine signaling to occur in EoE
[32]
on either elimination diet or fluticasone steroid . Collectively, both studies implicate miR-375 as an effec-
tive EoE biomarker, accurately reflecting disease activity [31,32] .
These findings were further substantiated by a study in 2014 that profiled miRNA expression in esophageal
[33]
[33]
biopsies of pediatric patients with EoE . Zahm et al. observed in their pediatric cohort that esophageal
expression of miR-375 was also lower in EoE individuals compared to healthy controls. Similar to adult stud-
ies, miR-375 expression inversely correlated with inflammation. Children with low miR-375 had increased
Th2 cytokines (IL-5 and IL-13) and mast-cell specific gene (CPA3 and TPSAB1) expression. Based on miR-
[34]
Walk2.0’s predictive algorithm, miR-375 is not expected to bind to IL-5, IL-13, CPA3 and TPSAB1 . As a
result, these Th2 cytokines are unlikely to be the direct targets of miR-375 activity and unlikely to constitute
the mechanism by which miR-375 regulates esophageal inflammation. It seems more likely based on predic-
tive algorithms that miR-375 regulates JAK2, a critical intercellular member of JAK/STAT-mediated cytokine
[33]
[34]
signaling pathways . Studies by Lu et al. [31,32] and Zahm et al. all similarly identify a deficiency of miR-
375 in diseased esophageal tissue that could, in theory, relieve inhibition on JAK2 and promote increased
signaling of Th2 cytokine pathways [Figure 3].
Abundance of miR-21 and miR-223 in esophageal epithelium associated with inflammation
[33]
[32]
In addition to miR-375, Zahm et al. and Lu et al. also found that miR-21, miR-223 were differentially
expressed in EoE tissue. In contrast to miR-375, both miR-21 and miR-223 were upregulated or present in
[32]
high levels in esophageal biopsies of EoE subjects. Lu et al. conducted genome-wide expression correlation
analysis which showed that miRNAs, miR-21 and miR-223, co-regulate genes involved in immune regula-
tion. More specifically, miR-21 and miR-223 control processes such as T cell polarization (Th1 vs. Th2), IFN-
[32]
gamma signaling and eosinophilia . miR-21 in particular has been shown in mouse models of allergic
inflammation and human disease to post-transcriptionally regulate a subunit of the IL-12 cytokine (IL-12p35).
By binding to the 3’ UTR of the IL-12p35 transcript, miR-21 can degrade IL-12-p35, inhibiting its action in
promotingTh1 immunity [Figure 4]. In this way, miR-21 inhibition of IL-12 shifts T cell polarization towards
[22]
Th2 immunity associated with allergic diseases . Consistent with this observation in allergic airway in-
[32]
flammation, Lu et al. also noted likely miR-21 inhibition of IL-12p35 in the esophageal biopsies of EoE pa-