Page 455 - Read Online
P. 455
Maner et al. J Cancer Metastasis Treat 2020;6:37 I http://dx.doi.org/10.20517/2394-4722.2020.60 Page 7 of 40
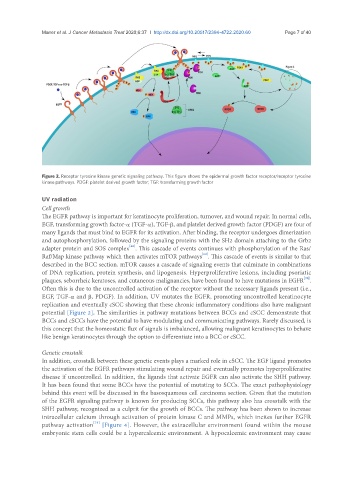
Figure 2. Receptor tyrosine kinase genetic signaling pathway. This figure shows the epidermal growth factor receptor/receptor tyrosine
kinase pathways. PDGF: platelet derived growth factor; TGF: transforming growth factor
UV radiation
Cell growth
The EGFR pathway is important for keratinocyte proliferation, turnover, and wound repair. In normal cells,
EGF, transforming growth factor-α (TGF-α), TGF-β, and platelet derived growth factor (PDGF) are four of
many ligands that must bind to EGFR for its activation. After binding, the receptor undergoes dimerization
and autophosphorylation, followed by the signaling proteins with the SH2 domain attaching to the Grb2
[68]
adapter protein and SOS complex . This cascade of events continues with phosphorylation of the Ras/
[69]
Raf/Map kinase pathway which then activates mTOR pathways . This cascade of events is similar to that
described in the BCC section. mTOR causes a cascade of signaling events that culminate in combinations
of DNA replication, protein synthesis, and lipogenesis. Hyperproliferative lesions, including psoriatic
[70]
plaques, seborrheic keratoses, and cutaneous malignancies, have been found to have mutations in EGFR .
Often this is due to the uncontrolled activation of the receptor without the necessary ligands present (i.e.,
EGF, TGF-α and β, PDGF). In addition, UV mutates the EGFR, promoting uncontrolled keratinocyte
replication and eventually cSCC showing that these chronic inflammatory conditions also have malignant
potential [Figure 2]. The similarities in pathway mutations between BCCs and cSCC demonstrate that
BCCs and cSCCs have the potential to have modulating and communicating pathways. Rarely discussed, is
this concept that the homeostatic flux of signals is imbalanced, allowing malignant keratinocytes to behave
like benign keratinocytes through the option to differentiate into a BCC or cSCC.
Genetic crosstalk
In addition, crosstalk between these genetic events plays a marked role in cSCC. The EGF ligand promotes
the activation of the EGFR pathways stimulating wound repair and eventually promotes hyperproliferative
disease if uncontrolled. In addition, the ligands that activate EGFR can also activate the SHH pathway.
It has been found that some BCCs have the potential of mutating to SCCs. The exact pathophysiology
behind this event will be discussed in the basosquamous cell carcinoma section. Given that the mutation
of the EGFR signaling pathway is known for producing SCCs, this pathway also has crosstalk with the
SHH pathway, recognized as a culprit for the growth of BCCs. The pathway has been shown to increase
intracellular calcium through activation of protein kinase C and MMPs, which incites further EGFR
pathway activation [Figure 4]. However, the extracellular environment found within the mouse
[71]
embryonic stem cells could be a hypercalcemic environment. A hypocalcemic environment may cause