Page 301 - Read Online
P. 301
Page 6 of 18 Qureshy et al. J Cancer Metastasis Treat 2020;6:27 I http://dx.doi.org/10.20517/2394-4722.2020.58
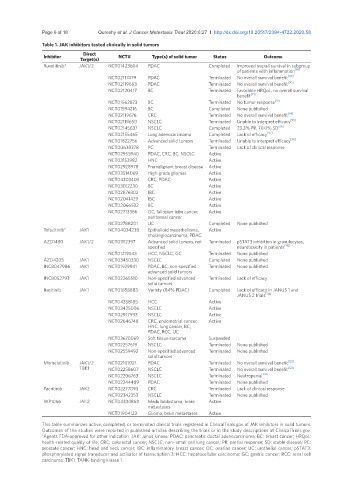
Table 1. JAK inhibitors tested clinically in solid tumors
Inhibitor Direct NCT# Type(s) of solid tumor Status Outcome
Target(s)
Ruxolitinib* JAK1/2 NCT01423604 PDAC Completed Improved overall survival in subgroup
of patients with inflammation [89]
NCT02117479 PDAC Terminated No overall survival benefit [90]
NCT02119663 PDAC Terminated No overall survival benefit [90]
NCT02120417 BC Terminated Favorable HRQoL, no overall survival
benefit [91]
NCT01562873 BC Terminated No tumor response [92]
NCT01594216 BC Completed None published
NCT02119676 CRC Terminated No overall survival benefit [94]
NCT02119650 NSCLC Terminated Unable to interpret efficacy [95]
NCT02145637 NSCLC Completed 23.3% PR, 70.0% SD [96]
NCT02155465 Lung adenocarcinoma Completed Lack of efficacy [97]
NCT01822756 Advanced solid tumors Terminated Unable to interpret efficacy [98]
NCT00638378 PC Terminated Lack of clinical response
NCT02955940 PDAC, CRC, BC, NSCLC Active
NCT03153982 HNC Active
NCT02928978 Premalignant breast disease Active
NCT03514069 High-grade gliomas Active
NCT04303403 CRC, PDAC Active
NCT03012230 BC Active
NCT02876302 IBC Active
NCT02041429 IBC Active
NCT02066532 BC Active
NCT02713386 OC, fallopian tube cancer, Active
peritoneal cancer
NCT02788201 UC Completed None published
Tofacitinib* JAK1 NCT04034238 Epithelioid mesothelioma, Active
cholangiocarcinoma, PDAC
AZD1480 JAK1/2 NCT01112397 Advanced solid tumors, not Terminated pSTAT3 inhibition in granulocytes,
specified neurotoxicity in patients [110]
NCT01219543 HCC, NSCLC, GC Terminated None published
AZD4205 JAK1 NCT03450330 NSCLC Completed None published
INCB047986 JAK1 NCT01929941 PDAC, BC, non-specified Terminated None published
advanced solid tumors
INCB052793 JAK1 NCT02265510 Non-specified advanced Terminated Lack of efficacy
solid tumors
Itacitinib JAK1 NCT01858883 Variety (84% PDAC) Completed Lack of efficacy in JANUS 1 and
JANUS 2 trials [114]
NCT04358185 HCC Active
NCT03425006 NSCLC Active
NCT02917993 NSCLC Active
NCT02646748 CRC, endometrial cancer, Active
HNC, lung cancer, BC,
PDAC, RCC, UC
NCT03670069 Soft tissue sarcoma Suspended
NCT02257619 NSCLC Terminated None published
NCT02559492 Non-specified advanced Terminated None published
solid tumors
Momelotinib JAK1/2 NCT02101021 PDAC Terminated No overall survival benefit [122]
TBK1 NCT02258607 NSCLC Terminated No overall survival benefit [123]
NCT02206763 NSCLC Terminated Neutropenia [124]
NCT02244489 PDAC Terminated None published
Pacritinib JAK2 NCT02277093 CRC Terminated Lack of clinical response
NCT02342353 NSCLC Terminated None published
WP1066 JAK2 NCT04334863 Medulloblastoma, brain Active
metastases
NCT01904123 Glioma, brain metastases Active
This table summarizes active, completed, or terminated clinical trials registered in ClinicalTrials.gov of JAK inhibitors in solid tumors.
Outcomes of the studies were reported in published articles describing the trials or in the study descriptions at ClinicalTrials.gov.
*Agents FDA-approved for other indication. JAK: Janus kinase; PDAC: pancreatic ductal adenocarcinoma; BC: breast cancer; HRQoL:
health-related quality of life; CRC: colorectal cancer; NSCLC: non- small cell lung cancer; PR: partial response; SD: stable disease; PC:
prostate cancer; HNC: head and neck cancer; IBC: inflammatory breast cancer; OC: ovarian cancer; UC: urothelial cancer; pSTAT3:
phosphorylated signal transducer and activator of transcription 3; HCC: hepatocellular carcinoma; GC: gastric cancer; RCC: renal cell
carcinoma; TBK1: TANK-binding kinase 1