Page 325 - Read Online
P. 325
Monks et al. J Cancer Metastasis Treat 2019;5:24 I http://dx.doi.org/10.20517/2394-4722.2018.79 Page 11 of 23
g
κ
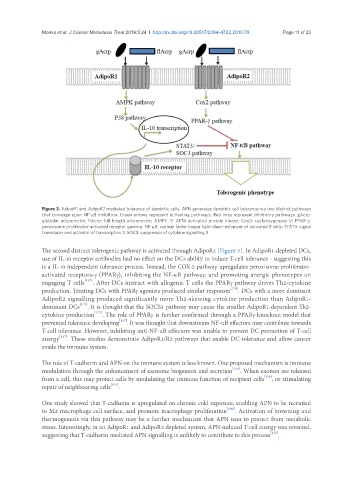
Figure 3. AdipoR1 and AdipoR2 mediated tolerance of dendritic cells. APN generates dendritic cell tolerance via two distinct pathways
that converge upon NF-κB inhibition. Green arrows represent activating pathways. Red lines represent inhibitory pathways. gAcrp:
globular adiponectin; flAcrp: full length adiponectin; AMPK: 5’ APM activated protein kinase; Cox2: cyclooxygenase-2; PPAR-g:
peroxisome proliferator-activated receptor gamma; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; STAT3: signal
transducer and activator of transcription 3; SOC3: suppressor of cytokine signalling 3
The second distinct tolerogenic pathway is activated through AdipoR2 [Figure 3]. In AdipoR1-depleted DCs,
use of IL-10 receptor antibodies had no effect on the DCs ability to induce T-cell tolerance - suggesting this
is a IL-10 independent tolerance process. Instead, the COX-2 pathway upregulates peroxisome proliferator-
activated receptors-g (PPARg), inhibiting the NF-κB pathway, and promoting anergic phenotypes on
engaging T cells [117] . After DCs interact with allogenic T cells the PPARg pathway drives Th2-cytokine
production. Treating DCs with PPARg agonists produced similar responses [176] . DCs with a more dominant
AdipoR2 signalling produced significantly more Th2-skewing cytokine production than AdipoR1-
dominant DCs [177] . It is thought that the SOCS3 pathway may cause the smaller AdipoR1-dependent Th2-
cytokine production [177] . The role of PPARg is further confirmed through a PPARg-knockout model that
prevented tolerance developing [117] . It was thought that downstream NF-κB effectors may contribute towards
T-cell tolerance. However, inhibiting anti-NF-κB effectors was unable to prevent DC promotion of T-cell
anergy [117] . These studies demonstrate AdipoR1/R2 pathways that enable DC-tolerance and allow cancer
evade the immune system.
The role of T-cadherin and APN on the immune system is less known. One proposed mechanism is immune
modulation through the enhancement of exosome biogenesis and secretion [124] . When exomes are released
from a cell, this may protect cells by modulating the immune function of recipient cells [178] , or stimulating
repair of neighbouring cells [179] .
One study showed that T-cadherin is upregulated on chronic cold exposure, enabling APN to be recruited
to M2 macrophage cell surface, and promote macrophage proliferation [180] . Activation of browning and
thermogenesis via this pathway may be a further mechanism that APN uses to protect from metabolic
stress. Interestingly, in an AdipoR1 and AdipoR2 depleted system, APN-induced T-cell anergy was reversed,
suggesting that T-cadherin mediated APN signalling is unlikely to contribute to this process [117] .