Page 583 - Read Online
P. 583
Ralph et al. J Cancer Metastasis Treat 2018;4:49 I http://dx.doi.org/10.20517/2394-4722.2018.42 Page 3 of 26
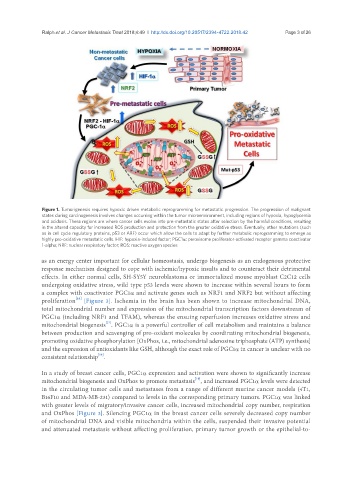
Figure 1. Tumorigenesis requires hypoxic driven metabolic reprogramming for metastatic progression. The progression of malignant
states during carcinogenesis involves changes occurring within the tumor microenvironment, including regions of hypoxia, hypoglycemia
and acidosis. These regions are where cancer cells evolve into pre-metastatic states after selection by the harmful conditions, resulting
in the altered capacity for increased ROS production and protection from the greater oxidative stress. Eventually, other mutations (such
as in cell cycle regulatory proteins, p53 or ARF) occur which allow the cells to adapt by further metabolic reprogramming to emerge as
highly pro-oxidative metastatic cells. HIF: hypoxia-induced factor; PGC1α: peroxisome proliferator-activated receptor gamma coactivator
1-alpha; NRF: nuclear respiratory factor; ROS: reactive oxygen species
as an energy center important for cellular homeostasis, undergo biogenesis as an endogenous protective
response mechanism designed to cope with ischemic/hypoxic insults and to counteract their detrimental
effects. In either normal cells, SH-SY5Y neuroblastoma or immortalized mouse myoblast C2C12 cells
undergoing oxidative stress, wild type p53 levels were shown to increase within several hours to form
a complex with coactivator PGC1α and activate genes such as NRF1 and NRF2 but without affecting
[16]
proliferation [Figure 3]. Ischemia in the brain has been shown to increase mitochondrial DNA,
total mitochondrial number and expression of the mitochondrial transcription factors downstream of
PGC1α (including NRF1 and TFAM), whereas the ensuing reperfusion increases oxidative stress and
[17]
mitochondrial biogenesis . PGC1α is a powerful controller of cell metabolism and maintains a balance
between production and scavenging of pro-oxidant molecules by coordinating mitochondrial biogenesis,
promoting oxidative phosphorylation [OxPhos, i.e., mitochondrial adenosine triphosphate (ATP) synthesis]
and the expression of antioxidants like GSH, although the exact role of PGC1α in cancer is unclear with no
[18]
consistent relationship .
In a study of breast cancer cells, PGC1α expression and activation were shown to significantly increase
[19]
mitochondrial biogenesis and OxPhos to promote metastasis , and increased PGC1α levels were detected
in the circulating tumor cells and metastases from a range of different murine cancer models (4T1,
B16F10 and MDA-MB-231) compared to levels in the corresponding primary tumors. PGC1α was linked
with greater levels of migratory/invasive cancer cells, increased mitochondrial copy number, respiration
and OxPhos [Figure 3]. Silencing PGC1α in the breast cancer cells severely decreased copy number
of mitochondrial DNA and visible mitochondria within the cells, suspended their invasive potential
and attenuated metastasis without affecting proliferation, primary tumor growth or the epithelial-to-