Page 16 - Read Online
P. 16
Page 6 of 9 Komiya et al. J Cancer Metastasis Treat 2018;4:1 I http://dx.doi.org/10.20517/2394-4722.2017.65
A B
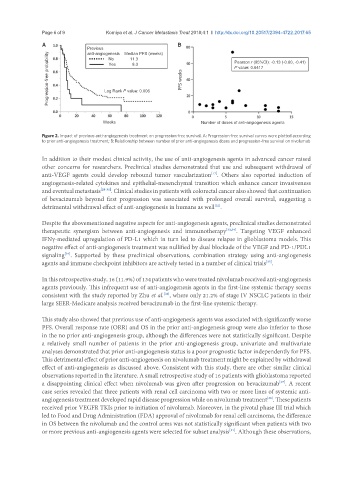
Previous No 11.3 Pearson r (95%CI): -0.13 (-0.60, -0.41)
anti-angiogenesis Median PFS (weeks)
Progression-free probability Log Rank P value: 0.006 PFS weeks P value: 0.6417
Yes
8.3
Weeks Number of doses of anti-angiogenesis agents
Figure 2. Impact of previous anti-angiogenesis treatment on progression-free survival. A: Progression-free survival curves were plotted according
to prior anti-angiogenesis treatment; B: Relationship between number of prior anti-angiogenesis doses and progression-free survival on nivolumab
In addition to their modest clinical activity, the use of anti-angiogenesis agents in advanced cancer raised
other concerns for researchers. Preclinical studies demonstrated that use and subsequent withdrawal of
[17]
anti-VEGF agents could develop rebound tumor vascularization . Others also reported induction of
angiogenesis-related cytokines and epithelial-mesenchymal transition which enhance cancer invasiveness
and eventual metastasis [28-31] . Clinical studies in patients with colorectal cancer also showed that continuation
of bevacizumab beyond first progression was associated with prolonged overall survival, suggesting a
detrimental withdrawal effect of anti-angiogenesis in humans as well .
[32]
Despite the abovementioned negative aspects for anti-angiogenesis agents, preclinical studies demonstrated
therapeutic synergism between anti-angiogenesis and immunotherapy [33,34] . Targeting VEGF enhanced
IFNγ-mediated upregulation of PD-L1 which in turn led to disease relapse in glioblastoma models. This
negative effect of anti-angiogenesis treatment was nullified by dual blockade of the VEGF and PD-1/PDL1
signaling . Supported by these preclinical observations, combination strategy using anti-angiogenesis
[34]
agents and immune checkpoint inhibitors are actively tested in a number of clinical trials .
[35]
In this retrospective study, 16 (11.9%) of 134 patients who were treated nivolumab received anti-angiogenesis
agents previously. This infrequent use of anti-angiogenesis agents in the first-line systemic therapy seems
consistent with the study reported by Zhu et al. , where only 21.2% of stage IV NSCLC patients in their
[36]
large SEER-Medicare analysis received bevacizumab in the first-line systemic therapy.
This study also showed that previous use of anti-angiogenesis agents was associated with significantly worse
PFS. Overall response rate (ORR) and OS in the prior anti-angiogenesis group were also inferior to those
in the no prior anti-angiogenesis group, although the differences were not statistically significant. Despite
a relatively small number of patients in the prior anti-angiogenesis group, univariate and multivariate
analyses demonstrated that prior anti-angiogenesis status is a poor prognostic factor independently for PFS.
This detrimental effect of prior anti-angiogenesis on nivolumab treatment might be explained by withdrawal
effect of anti-angiogenesis as discussed above. Consistent with this study, there are other similar clinical
observations reported in the literature. A small retrospective study of 16 patients with glioblastoma reported
[37]
a disappointing clinical effect when nivolumab was given after progression on bevacizumab . A recent
case series revealed that three patients with renal cell carcinoma with two or more lines of systemic anti-
[38]
angiogenesis treatment developed rapid disease progression while on nivolumab treatment . These patients
received prior VEGFR TKIs prior to initiation of nivolumab. Moreover, in the pivotal phase III trial which
led to Food and Drug Administration (FDA) approval of nivolumab for renal cell carcinoma, the difference
in OS between the nivolumab and the control arms was not statistically significant when patients with two
[11]
or more previous anti-angiogenesis agents were selected for subset analysis . Although these observations,