Page 14 - Read Online
P. 14
Page 4 of 9 Komiya et al. J Cancer Metastasis Treat 2018;4:1 I http://dx.doi.org/10.20517/2394-4722.2017.65
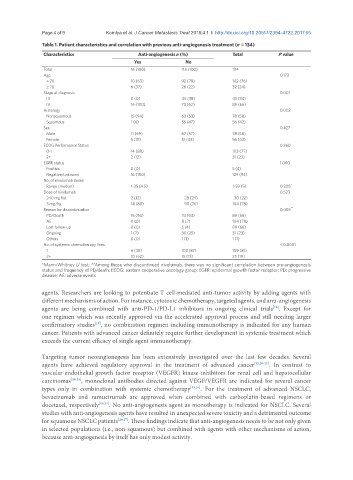
Table 1. Patient characteristics and correlation with previous anti-angiogenesis treatment (n = 134)
Characteristics Anti-angiogenesis n (%) Total P value
Yes No
Total 16 (100) 118 (100) 134
Age 0.173
< 70 10 (63) 92 (78) 102 (76)
≥ 70 6 (37) 26 (22) 32 (24)
Stage at diagnosis 0.001
III 0 (0) 45 (38) 45 (34)
IV 16 (100) 73 (62) 89 (66)
Histology 0.002
Nonsquamous 15 (94) 63 (53) 78 (58)
Squamous 1 (6) 55 (47) 56 (42)
Sex 0.427
Male 11 (69) 67 (57) 78 (58)
Female 5 (31) 51 (43) 56 (42)
ECOG Performance Status 0.360
0-1 14 (88) 103 (77)
2+ 2 (12) 31 (23)
EGFR status 1.000
Positive 0 (0) 5 (4)
Negative/unknown 16 (100) 129 (94)
No. of nivolumab doses
Range (median) 1-35 (4.5) 1-59 (5) 0.208 *
Dose of nivolumab 0.523
240 mg flat 2 (12) 28 (24) 30 (22)
3 mg/kg 14 (88) 90 (76) 104 (78)
Reason for discontinuation 0.408 **
PD/Death 15 (94) 74 (63) 89 (66)
AE 0 (0) 8 (7) 104 (78)
Lost follow-up 0 (0) 5 (4) 89 (66)
Ongoing 1 (7) 30 (25) 31 (23)
Others 0 (0) 1 (1) 1 (1)
No. of systemic chemotherapy lines < 0.0001
1 6 (38) 103 (87) 109 (81)
2+ 10 (62) 15 (13) 25 (19)
*Mann-Whitney U test; **Among those who discontinued nivolumab, there was no significant correlation between pre-angiogenesis
status and frequency of PD/death. ECOG: eastern cooperative oncology group; EGFR: epidermal growth factor receptor; PD: progressive
disease; AE: adverse events
agents. Researchers are looking to potentiate T cell-mediated anti-tumor activity by adding agents with
different mechanisms of action. For instance, cytotoxic chemotherapy, targeted agents, and anti-angiogenesis
agents are being combined with anti-PD-1/PD-L1 inhibitors in ongoing clinical trials . Except for
[16]
one regimen which was recently approved via the accelerated approval process and still needing larger
confirmatory studies , no combination regimen including immunotherapy is indicated for any human
[19]
cancer. Patients with advanced cancer definitely require further development in systemic treatment which
exceeds the current efficacy of single agent immunotherapy.
Targeting tumor neoangionegesis has been extensively investigated over the last few decades. Several
agents have achieved regulatory approval in the treatment of advanced cancer [15,20-25] . In contrast to
vascular endothelial growth factor receptor (VEGFR) kinase inhibitors for renal cell and hepatocellular
carcinomas [20-23] , monoclonal antibodies directed against VEGF/VEGFR are indicated for several cancer
types only in combination with systemic chemotherapy [15,24] . For the treatment of advanced NSCLC,
bevacizumab and ramucirumab are approved when combined with carboplatin-based regimens or
docetaxel, respectively [15,24] . No anti-angiogenesis agent as monotherapy is indicated for NSCLC. Several
studies with anti-angiogenesis agents have resulted in unexpected severe toxicity and a detrimental outcome
for squamous NSCLC patients [26,27] . These findings indicate that anti-angiogenesis needs to be not only given
in selected populations (i.e., non-squamous) but combined with agents with other mechanisms of action,
because anti-angiogenesis by itself has only modest activity.