Page 53 - Read Online
P. 53
its spatial, functional and morphological manifestations. it hampers the efficacy of current cancer treatments. It is
However, in order to translate this still growing knowledge triggered by the transient or permanent induction of motility
into clinical applications targeting the tumor phenotype, and invasiveness in the tumor cells. An essential prerequisite
sophisticated model systems are necessary to explore and for primary brain tumor cell migration and invasion is the
validate potential interference strategies under physiologically remodeling of the actin and tubulin cytoskeletons, [7-9] which
relevant conditions. In addition, functional genomics and not only provide force, traction and rigidity but also scaffold
cell-based molecular analyses are indispensable in many signaling complexes in a spatially controlled manner. [10-12]
cases to clarify whether mutated or amplified genes are Hence, blocking motility and invasiveness by targeting
necessarily contributory to an altered proteome and causative pro-migratory cytoskeleton dynamics in tumor cells could
for the cancerous phenotype. Moreover, the current wealth of prevent local tumor cell invasion, further dissemination
genomic and transcriptomic data is insufficient on its own to from proximal metastases and the evolution towards a
isolate specific signaling networks driving tumor progression more aggressive phenotype. In a seminal review by Giese
from a benign lesion to a disseminated cancer. Hence, to et al., the dichotomy of migration and proliferation in
[13]
tackle the complexity of the metastatic process it is necessary gliomas was recognized as the consequence of antagonistic
to dissect it into individual steps that can be addressed with cell regulation. Consequently, the authors concluded that
rationally adapted model systems. In this review we focus an approach to influence the underlying mechanisms could
on in vitro and ex vivo primary brain tumor model systems be the basis of novel anti-invasive therapy strategies. A
and discuss how they can be improved and used to develop computational modeling study predicts that even a small
the molecular understanding necessary for designing novel
anti-metastatic therapies. While none of these model systems increase in the motile capability of tumor cells, and the
consequent short-range dissemination, increases net tumor
on its own will suffice to tackle such a complex disease as [14]
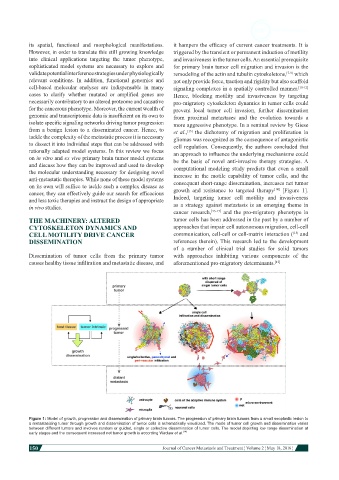
cancer, they can effectively guide our search for efficacious growth and resistance to targeted therapy [Figure 1].
and less toxic therapies and instruct the design of appropriate Indeed, targeting tumor cell motility and invasiveness
in vivo studies. as a strategy against metastasis is an emerging theme in
cancer research, [15-17] and the pro-migratory phenotype in
THE MACHINERY: ALTERED tumor cells has been addressed in the past by a number of
CYTOSKELETON DYNAMICS AND approaches that impair cell autonomous migration, cell-cell
[15]
CELL MOTILITY DRIVE CANCER communication, cell-cell or cell-matrix interaction ( and
DISSEMINATION references therein). This research led to the development
of a number of clinical trial studies for solid tumors
Dissemination of tumor cells from the primary tumor with approaches inhibiting various components of the
causes healthy tissue infiltration and metastatic disease, and aforementioned pro-migratory determinants. [15]
Figure 1: Model of growth, progression and dissemination of primary brain tumors. The progression of primary brain tumors from a small neoplastic lesion to
a metastasizing tumor through growth and dissemination of tumor cells is schematically visualized. The mode of tumor cell growth and dissemination varies
between different tumors and involves random or guided, single or collective dissemination of tumor cells. The model depicting low range dissemination at
early stages and the consequent increased net tumor growth is according Waclaw et al. [14]
150
Journal of Cancer Metastasis and Treatment ¦ Volume 2 ¦ May 18, 2016 ¦