Page 8 - Read Online
P. 8
Fitzgerald et al. J Cancer Metastasis Treat 2021;7:54 https://dx.doi.org/10.20517/2394-4722.2021.97 Page 3 of 11
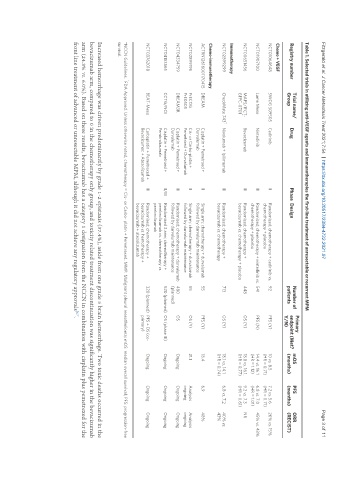
Table 1. Selected trials in utilizing anti-VEGF agents and immunotherapies the first-line treatment of unresectable or recurrent MPM
Primary
Trial name/ Number of mOS PFS ORR
Registry number Drug Phase Design endpoint (Met?
Group patients Y/N) (months) (months) (RECIST)
Chemo + VEGF
NCT01064648 SWOG S09505 Cedirinib II Randomized; chemotherapy + cedirinib vs. 92 PFS (Y) 10 vs. 8.5 7.2 vs. 5.6 26% vs. 15%
chemotherapy + placebo (HR = 0.71) (HR = 0.71)
NCT01907100 Lume Meso Nintedinib III Randomized; chemotherapy + nintedinib vs. 541 PFS (N) 14.4 vs. 16.1 6.8 vs. 7.0 45% vs. 43%
chemotherapy + placebo (HR = 1.12) (HR = 1.01)
NCT00651456 MAPS/IFCT- Bevacizumab III Randomized; chemotherapy + 448 OS (Y) 18.8 vs. 16.1. 9.2 vs. 7.3 NR
GFPC-0701 * bevacizumab vs. chemotherapy + placebo (HR = 0.77) (HR = 0.61)
Immunotherapy
NCT02899299 CheckMate 743 *, Nivolumab + Ipilimumab III Randomized; chemotherapy + 713 OS (Y) 18.1 vs. 14.1 6.8 vs. 7.2 40% vs.
^
bevacizumab vs. chemotherapy (HR = 0.74) 43%
Chemo-immunotherapy
ACTRN12616001170415 DREAM Cisplatin + Pemetrxed + II Single arm; chemotherapy + durvalumab 55 PFS (Y) 18.4 6.9 48%
Durvalumab followed by durvalumab maintenance
NCT02899195 PrECOG Cis- or Carbo-platin + II Single arm; chemotherapy + durvalumab 55 OS (Y) 21.1 Analysis Analysis
PrE0505 Pemetrxed + Durvalumab followed by durvalumab maintenance ongoing ongoing
NCT04334759 DREAM3R Cisplatin + Pemetrxed + III Randomised; chemotherapy + durvalumab 480 OS Ongoing Ongoing Ongoing
Durvalumab followed by durvalumab maintenance (planned)
NCT04153565 CCTG/NCI Cisplatin + Pemetrxed + II/III Randomised 3 arm; chemotherapy + 520 (planned) OS (phase III) Ongoing Ongoing Ongoing
Pembrolizumab pembrolizumab vs. chemotherapy vs.
pembrolizumab
NCT03762018 BEAT-Meso Carboplatin + Pemetrexed + III Randomised; chemotherapy + 320 (planned) PFS + OS (co- Ongoing Ongoing Ongoing
Bevacizumac + Atezolizumab bevacizumab vs. hemotherapy + primary)
bevacizumab + atezolizumab
^
*NCCN Guidelines. FDA Approved. Unless otherwise noted, chemotherapy = Cis- or Carbo- platin + Pemetrexed. MMP: Malignant pleural mesothelioma; mOS: median overall survival; PFS: progression-free
survival.
Increased hemorrhage was driven predominantly by grade 1-2 epistaxis (37.4%), aside from one grade 5 brain hemorrhage. Two toxic deaths occurred in the
bevacizumab arm, compared to 0 in the chemotherapy only group, and toxicity related treatment discontinuation was significantly higher in the bevacizumab
arm (24.3% vs. 6.0%). Based on these results, bevacizumab has a category 1 designation from the NCCN in combination with cisplatin plus pemetrexed for the
front line treatment of advanced or unresectable MPM, although it did not achieve any regulatory approvals .
[6,7]