Page 12 - Read Online
P. 12
Fitzgerald et al. J Cancer Metastasis Treat 2021;7:54 https://dx.doi.org/10.20517/2394-4722.2021.97 Page 7 of 11
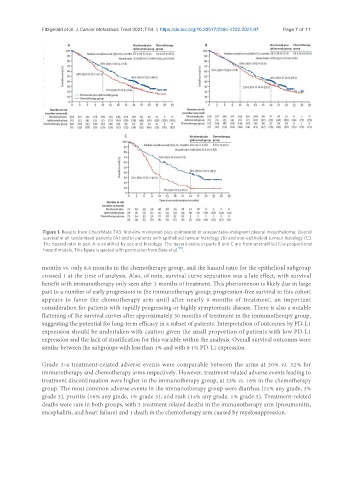
Figure 1. Results from CheckMate 743: first-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma. Overall
survival in all randomised patients (A) and in patients with epithelioid tumour histology (B) and non-epithelioid tumour histology (C).
The hazard ratio in part A is stratified by sex and histology. The hazard ratios in parts B and C are from unstratified Cox proportional
hazard models. This figure is quoted with permission from Baas et al. [10] .
months vs. only 8.8 months in the chemotherapy group, and the hazard ratio for the epithelioid subgroup
crossed 1 at the time of analysis. Also, of note, survival curve separation was a late effect, with survival
benefit with immunotherapy only seen after 3 months of treatment. This phenomenon is likely due in large
part to a number of early progressors in the immunotherapy group; progression-free survival in this cohort
appears to favor the chemotherapy arm until after nearly 9 months of treatment, an important
consideration for patients with rapidly progressing or highly symptomatic disease. There is also a notable
flattening of the survival curves after approximately 30 months of treatment in the immunotherapy group,
suggesting the potential for long-term efficacy in a subset of patients. Interpretation of outcomes by PD-L1
expression should be undertaken with caution given the small proportion of patients with low PD-L1
expression and the lack of stratification for this variable within the analysis. Overall survival outcomes were
similar between the subgroups with less than 1% and with ≥ 1% PD-L1 expression.
Grade 3-4 treatment-related adverse events were comparable between the arms at 30% vs. 32% for
immunotherapy and chemotherapy arms respectively. However, treatment related adverse events leading to
treatment discontinuation were higher in the immunotherapy group, at 23% vs. 16% in the chemotherapy
group. The most common adverse events in the immunotherapy group were diarrhea (21% any grade, 3%
grade 3), pruritis (16% any grade, 1% grade 3), and rash (14% any grade, 1% grade 3). Treatment-related
deaths were rare in both groups, with 3 treatment related deaths in the immunotherapy arm (pneumonitis,
encephalitis, and heart failure) and 1 death in the chemotherapy arm caused by myelosuppression.