Page 46 - Read Online
P. 46
Cote et al. J Cancer Metastasis Treat 2022;8:36 https://dx.doi.org/10.20517/2394-4722.2022.41 Page 3 of 11
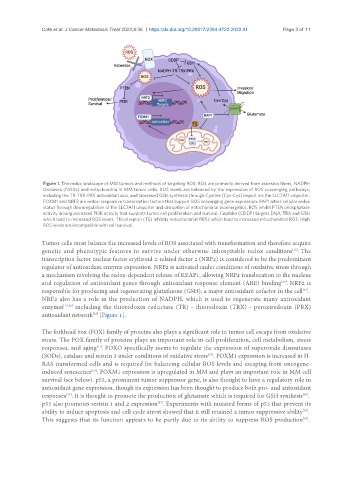
Figure 1. The redox landscape of MM tumors and methods of targeting ROS: ROS are primarily derived from asbestos fibers, NADPH
Oxidases (NOXs) and mitochondria in MM tumor cells. ROS levels are balanced by the expression of ROS scavenging pathways,
including the TR-TRX-PRX antioxidant axis, and increased GSH synthesis through Cystine (Cys-Cys) import via the SLC7A11 uniporter.
FOXM1 and NRF2 are redox-responsive transcription factors that support ROS scavenging gene expression. BAP1 alters cellular redox
status through downregulation of the SLC7A11 uniporter and disruption of mitochondrial bioenergetics. ROS inhibit PTEN phosphatase
activity driving increased PI3K activity that supports tumor cell proliferation and survival. Cisplatin (CDDP) targets DNA, TRX and GSH
which lead to increased ROS levels. Thiostrepton (TS) inhibits mitochondrial PRX3 which lead to increased mitochondrial ROS. High
ROS levels are incompatible with cell survival.
Tumor cells must balance the increased levels of ROS associated with transformation and therefore acquire
genetic and phenotypic features to survive under otherwise inhospitable redox conditions . The
[29]
transcription factor nuclear factor erythroid 2-related factor 2 (NRF2) is considered to be the predominant
regulator of antioxidant enzyme expression. NRF2 is activated under conditions of oxidative stress through
a mechanism involving the redox-dependent release of KEAP1, allowing NRF2 translocation to the nucleus
and regulation of antioxidant genes through antioxidant response element (ARE) binding . NRF2 is
[30]
responsible for producing and regenerating glutathione (GSH), a major antioxidant cofactor in the cell .
[31]
NRF2 also has a role in the production of NADPH, which is used to regenerate many antioxidant
enzymes [17,32] including the thioredoxin reductase (TR) - thioredoxin (TRX) - peroxiredoxin (PRX)
[33]
antioxidant network [Figure 1].
The forkhead box (FOX) family of proteins also plays a significant role in tumor cell escape from oxidative
stress. The FOX family of proteins plays an important role in cell proliferation, cell metabolism, stress
responses, and aging . FOXO specifically seems to regulate the expression of superoxide dismutases
[17]
(SODs), catalase and sestrin 3 under conditions of oxidative stress . FOXM1 expression is increased in H-
[34]
RAS transformed cells and is required for balancing cellular ROS levels and escaping from oncogene-
induced senescence . FOXM1 expression is upregulated in MM and plays an important role in MM cell
[25]
survival (see below). p53, a prominent tumor suppressor gene, is also thought to have a regulatory role in
antioxidant gene expression, though its expression has been thought to produce both pro- and antioxidant
responses . It is thought to promote the production of glutamate which is required for GSH synthesis .
[36]
[35]
p53 also promotes sestrin 1 and 2 expression . Experiments with mutated forms of p53 that prevent its
[37]
ability to induce apoptosis and cell cycle arrest showed that it still retained a tumor suppressive ability .
[38]
[39]
This suggests that its function appears to be partly due to its ability to suppress ROS production .