Page 27 - Read Online
P. 27
Fujimoto et al. J Cancer Metastasis Treat 2021;7:66 https://dx.doi.org/10.20517/2394-4722.2021.157 Page 9 of 14
Table 2. Clinical trials of single novel agent for relapsed or refractory ENKL patients
Agent Target Trial phase n ORR CR rate Ref.
Pembrolizumab PD-1 Retrospective 7 100 71 [71]
Pembrolizumab PD-1 Retrospective 19 47 37 [72]
Pembrolizumab PD-1 Retrospective 7 57 29 [73]
Nivolumab PD-1 Retrospective 3 67 67 [74]
Sintilimab PD-1 Phase II 28 68 14 [75]
Avelumab PD-L1 Phase II 21 38 24 [76]
Brentuximab vedotin CD30 Phase II 7 29 15 [82]
Daratumumab CD38 Phase II 32 25 0 [83]
Chidamide HDAC Phase II 79 (16) 19 6 [85]
LMP1/2-specific CTL LMP1/2 Phase II 6 67 67 [87]
HDAC: Histone deacetylase; LMP: latent membrane protein; CTL: cytotoxic T-cells; NA: not available; ORR: overall response rate; CR: complete
response; ENKL: extranodal NK/T cell lymphoma, nasal type.
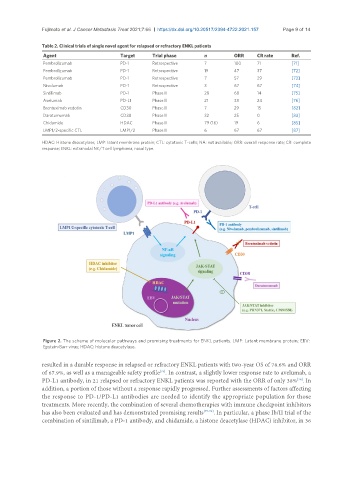
Figure 2. The scheme of molecular pathways and promising treatments for ENKL patients. LMP: Latent membrane protein; EBV:
Epstein-Barr virus; HDAC: histone deacetylase.
resulted in a durable response in relapsed or refractory ENKL patients with two-year OS of 78.6% and ORR
[75]
of 67.9%, as well as a manageable safety profile . In contrast, a slightly lower response rate to avelumab, a
[76]
PD-L1 antibody, in 21 relapsed or refractory ENKL patients was reported with the ORR of only 38% . In
addition, a portion of those without a response rapidly progressed. Further assessments of factors affecting
the response to PD-1/PD-L1 antibodies are needed to identify the appropriate population for those
treatments. More recently, the combination of several chemotherapies with immune checkpoint inhibitors
has also been evaluated and has demonstrated promising results [77-79] . In particular, a phase Ib/II trial of the
combination of sintilimab, a PD-1 antibody, and chidamide, a histone deacetylase (HDAC) inhibitor, in 36