Page 24 - Read Online
P. 24
Page 6 of 14 Fujimoto et al. J Cancer Metastasis Treat 2021;7:66 https://dx.doi.org/10.20517/2394-4722.2021.157
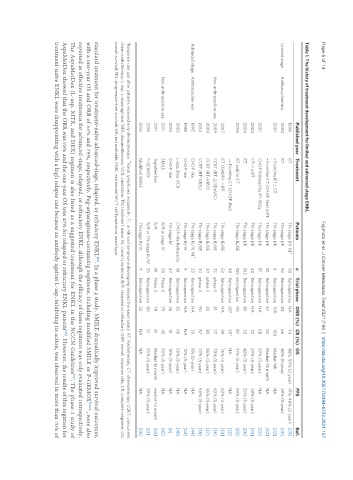
Table 1. The history of treatment development for limited and advanced stage ENKL
Published year Treatment Patients n Trial phase ORR (%) CR (%) OS PFS Ref.
1998 RT TN-stage I/II NL + 90 Retrospective NA 74 48%-59% (2-year) 41%-48% (2-year) [24]
Limited stage Anthracycline-era 2000 RT TN-stage I/II 92 Retrospective 84 66 40% (5-year) 38% (5-year) [25]
2001 • Frontline RT ± CT TN-stage I/II 8 Retrospective 100 100 Median NR NA [32]
• Frontline CT (CHOP-like) ± RT TN-stage I/II 12 Retrospective 67 25 Median 35 month NA [32]
2001 CHOP followed by RT 45Gy TN-stage I/II 17 Retrospective NA 58 59% (3-year) NA [33]
2004 CT-> ± RT TN-stage I/II 40 Retrospective NA 72 29% (5-year) 28% (5-year) [34]
2004 RT TN-stage I/II 102 Retrospective 85 72 42% (5-year) 53% (5-year) [26]
2006 RT and/or CT TN-stage IE/IIE 105 retrospective 90 87 71% (5-year) 59% (5-year) [22]
→ Frontline CT (CHOP-like) 40 Retrospective 60* 20* NA NA [22]
2007 CT (CHOP) -> RT TN-stage IE/IIE 53 Retrospective NA 49 76% (2-year) 62% (2-year) [34]
Non-anthracycline-era 2009 CCRT (RT-2/3DeVIC) TN-stage IE/IIE 33 phase II 81 77 70% (5-year) 63% (5-year) [36]
2009 CCRT (RT-VIPD) TN-stage IE/IIE 30 phase II 83 80 86% (3-year) 85% (3-year) [37]
2014 CCRT (RT-VIDL) TN-stage IE/IIE 30 phase II 90 87 73% (5-year) 60% (5-year) [38]
+
Advanced stage Anthracycline-era 1995 CHOP-like TN-stage III/IV NL 33 Retrospective NA 30 8% (5-year) NA [44]
1998 CHOP-like TN-stage III/IV 9 Retrospective NA NA 12% (5-year) NA [24]
2003 L-asp, Dex, VCR CHOP-like Refractory 18 Retrospective 83 56 56% (5-year) NA [48]
2010 CHOP-like TN-stage IV 47 Retrospective 36 10 10% (5-year) NA [5]
Non-anthracycline-era 2011 SMILE R/R or stage IV 38 Phase II 79 45 50% (5-year) NA [42]
2011 AspaMetDex R/R 19 Phase II 78 61 Median 12 month median 12 month [53]
2016 P-GEMOX R/R or TN-stage III/IV 35 Retrospective 80 51 65% (3-year) 39% (3-year) [51]
2016 Modified SMILE TN-stage III/IV 9 Retrospective 22 NA NA NA [50]
+
*Response rate just after patients received only chemotherapy. Nasal lymphoma includes B-, T-, or NK-cell lymphoma developing around the nasal cavity. RT: Radiotherapy; CT: chemotherapy; CCRT: concurrent
chemoradiotherapy; L-asp: L-asparaginase; DEX: dexamethasone; VCR: vincristine; TN: treatment-naive; NL: nasal lymphoma; R/R: relapsed or refractory; ORR: overall response rate; CR: complete response; OS:
overall survival; PFS: progression-free survival; NA: not available; ENKL: extranodal NK/T cell lymphoma, nasal type.
standard treatment for treatment-naïve advanced-stage, relapsed, or refractory ENKL . In a phase 2 study, SMILE dramatically improved survival outcomes,
[42]
with a one-year OS and ORR of 55% and 79%, respectively. Peg-asparaginase-containing regimens, including modified SMILE or P-GEMOX [50,51] , were also
reported as effective treatments for advanced-stage, relapsed, or refractory ENKL, although the efficacy of these regimens was only evaluated retrospectively.
[52]
The AspaMetDex (L-asp, MTX, and DEX) regimen is also listed as a suggested treatment for ENKL in the NCCN Guidelines . The phase 2 study of
[53]
AspaMetDex showed that the ORR was 78% and the one-year OS was 47% for relapsed or refractory ENKL patients . However, the results of this regimen for
treatment-naïve ENKL were disappointing with a high relapse rate because an antibody against L-asp, inhibiting its action, was observed in more than 70% of