Page 40 - Read Online
P. 40
Tosato et al. J Cancer Metastasis Treat 2021;7:52 https://dx.doi.org/10.20517/2394-4722.2021.120 Page 3 of 14
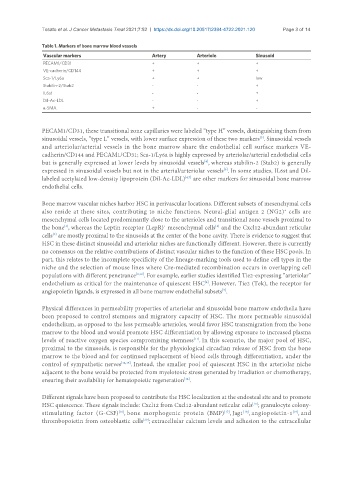
Table 1. Markers of bone marrow blood vessels
Vascular markers Artery Arteriole Sinusoid
PECAM1/CD31 + + +
VE-cadherin/CD144 + + +
Sca-1/Ly6a + + low
Stabilin-2/Stab2 - - +
IL6st - - +
Dil-Ac-LDL - - +
α-SMA + - -
PECAM1/CD31, these transitional zone capillaries were labeled “type H” vessels, distinguishing them from
sinusoidal vessels, “type L” vessels, with lower surface expression of these two markers . Sinusoidal vessels
[5]
and arteriolar/arterial vessels in the bone marrow share the endothelial cell surface markers VE-
cadherin/CD144 and PECAM1/CD31; Sca-1/Ly6a is highly expressed by arteriolar/arterial endothelial cells
but is generally expressed at lower levels by sinusoidal vessels , whereas stabilin-2 (Stab2) is generally
[4]
[6]
expressed in sinusoidal vessels but not in the arterial/arteriolar vessels . In some studies, IL6st and Dil-
labeled acetylated low-density lipoprotein (Dil-Ac-LDL) are other markers for sinusoidal bone marrow
[4,7]
endothelial cells.
Bone marrow vascular niches harbor HSC in perivascular locations. Different subsets of mesenchymal cells
+
also reside at these sites, contributing to niche functions. Neural-glial antigen 2 (NG2) cells are
mesenchymal cells located predominantly close to the arterioles and transitional zone vessels proximal to
the bone , whereas the Leptin receptor (LepR) mesenchymal cells and the Cxcl12-abundant reticular
[4]
+
[8]
[9]
cells are mostly proximal to the sinusoids at the center of the bone cavity. There is evidence to suggest that
HSC in these distinct sinusoidal and arteriolar niches are functionally different. However, there is currently
no consensus on the relative contributions of distinct vascular niches to the function of these HSC pools. In
part, this relates to the incomplete specificity of the lineage-marking tools used to define cell types in the
niche and the selection of mouse lines where Cre-mediated recombination occurs in overlapping cell
populations with different penetrance [3,10] . For example, earlier studies identified Tie2-expressing “arteriolar”
endothelium as critical for the maintenance of quiescent HSC . However, Tie2 (Tek), the receptor for
[4]
angiopoietin ligands, is expressed in all bone marrow endothelial subsets .
[7]
Physical differences in permeability properties of arteriolar and sinusoidal bone marrow endothelia have
been proposed to control stemness and migratory capacity of HSC. The more permeable sinusoidal
endothelium, as opposed to the less permeable arterioles, would favor HSC transmigration from the bone
marrow to the blood and would promote HSC differentiation by allowing exposure to increased plasma
levels of reactive oxygen species compromising stemness . In this scenario, the major pool of HSC,
[11]
proximal to the sinusoids, is responsible for the physiological circadian release of HSC from the bone
marrow to the blood and for continued replacement of blood cells through differentiation, under the
control of sympathetic nerves [12,13] . Instead, the smaller pool of quiescent HSC in the arteriolar niche
adjacent to the bone would be protected from myelotoxic stress generated by irradiation or chemotherapy,
ensuring their availability for hematopoietic regeneration .
[14]
Different signals have been proposed to contribute the HSC localization at the endosteal site and to promote
HSC quiescence. These signals include: Cxcl12 from Cxcl12-abundant reticular cells ; granulocyte colony-
[15]
[16]
[19]
[18]
stimulating factor (G-CSF) , bone morphogenic protein (BMP) , Jag1 , angiopoietin-1 , and
[17]
[20]
thrombopoietin from osteoblastic cells ; extracellular calcium levels and adhesion to the extracellular