Page 42 - Read Online
P. 42
Tosato et al. J Cancer Metastasis Treat 2021;7:52 https://dx.doi.org/10.20517/2394-4722.2021.120 Page 5 of 14
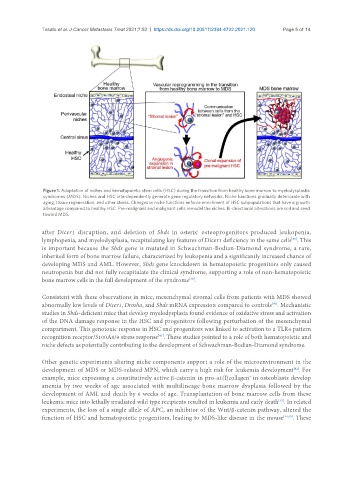
Figure 1. Adaptation of niches and hematopoietic stem cells (HSC) during the transition from healthy bone marrow to myelodysplastic
syndromes (MDS). Niches and HSC interdependently generate gene regulatory networks. Niche functions gradually deteriorate with
aging, tissue regeneration, and other stress. Changes in niche functions enforce enrichment of HSC subpopulations that have a growth
advantage compared to healthy HSC. Pre-malignant and malignant cells remodel the niches. Bi-directional alterations are soil and seed
toward MDS.
after Dicer1 disruption, and deletion of Sbds in osterix osteoprogenitors produced leukopenia,
+
lymphopenia, and myelodysplasia, recapitulating key features of Dicer1 deficiency in the same cells . This
[39]
is important because the Sbds gene is mutated in Schwachman-Bodian-Diamond syndrome, a rare,
inherited form of bone marrow failure, characterized by leukopenia and a significantly increased chance of
developing MDS and AML. However, Sbds gene knockdown in hematopoietic progenitors only caused
neutropenia but did not fully recapitulate the clinical syndrome, supporting a role of non-hematopoietic
bone marrow cells in the full development of the syndrome .
[39]
Consistent with these observations in mice, mesenchymal stromal cells from patients with MDS showed
abnormally low levels of Dicer1, Drosha, and Sbds mRNA expression compared to controls . Mechanistic
[40]
studies in Sbds-deficient mice that develop myelodysplasia found evidence of oxidative stress and activation
of the DNA damage response in the HSC and progenitors following perturbation of the mesenchymal
compartment. This genotoxic response in HSC and progenitors was linked to activation to a TLR4 pattern
[41]
recognition receptor/S100A8/9 stress response . These studies pointed to a role of both hematopoietic and
niche defects as potentially contributing to the development of Schwachman-Bodian-Diamond syndrome.
Other genetic experiments altering niche components support a role of the microenvironment in the
[42]
development of MDS or MDS-related MPN, which carry a high risk for leukemia development . For
example, mice expressing a constitutively active β-catenin in pro-α1(I)collagen in osteoblasts develop
+
anemia by two weeks of age associated with multilineage bone marrow dysplasia followed by the
development of AML and death by 6 weeks of age. Transplantation of bone marrow cells from these
leukemic mice into lethally irradiated wild type recipients resulted in leukemia and early death . In related
[43]
experiments, the loss of a single allele of APC, an inhibitor of the Wnt/β-catenin pathway, altered the
function of HSC and hematopoietic progenitors, leading to MDS-like disease in the mouse [44,45] . These