Page 39 - Read Online
P. 39
Page 6 of 19 Matrone et al. J Cancer Metastasis Treat 2021;7:23 https://dx.doi.org/10.20517/2394-4722.2021.47
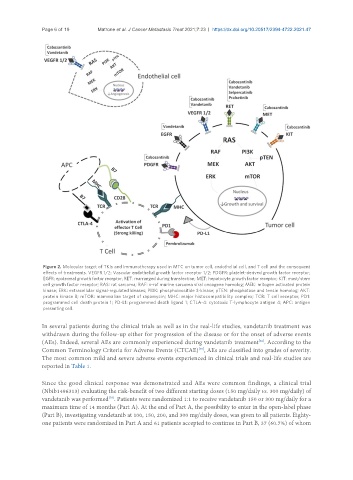
Figure 2. Molecular target of TKIs and immunotherapy used in MTC on tumor cell, endothelial cell, and T cell and the consequent
effects of treatments. VEGFR 1/2: Vascular endothelial growth factor receptor 1/2; PDGFR: platelet-derived growth factor receptor;
EGFR: epidermal growth factor receptor; RET: rearranged during transfection; MET: hepatocyte growth factor receptor; KIT: mast/stem
cell growth factor receptor; RAS: rat sarcoma; RAF: v-raf murine sarcoma viral oncogene homolog; MEK: mitogen activated protein
kinase; ERK: extracellular signal-regulated kinases; PI3K: phosphoinositide 3-kinase; pTEN: phosphatase and tensin homolog; AKT:
protein kinase B; mTOR: mammalian target of rapamycin; MHC: major histocompatibility complex; TCR: T cell receptor; PD1:
programmed cell death protein 1; PD-L1: programmed death ligand 1; CTLA-4: cytotoxic T-lymphocyte antigen 4; APC: antigen
presenting cell.
In several patients during the clinical trials as well as in the real-life studies, vandetanib treatment was
withdrawn during the follow-up either for progression of the disease or for the onset of adverse events
(AEs). Indeed, several AEs are commonly experienced during vandetanib treatment . According to the
[66]
Common Terminology Criteria for Adverse Events (CTCAE) , AEs are classified into grades of severity.
[75]
The most common mild and severe adverse events experienced in clinical trials and real-life studies are
reported in Table 1.
Since the good clinical response was demonstrated and AEs were common findings, a clinical trial
(Nbib1496313) evaluating the risk-benefit of two different starting doses (150 mg/daily vs. 300 mg/daily) of
vandetanib was performed . Patients were randomized 1:1 to receive vandetanib 150 or 300 mg/daily for a
[78]
maximum time of 14 months (Part A). At the end of Part A, the possibility to enter in the open-label phase
(Part B), investigating vandetanib at 100, 150, 200, and 300 mg/daily doses, was given to all patients. Eighty-
one patients were randomized in Part A and 61 patients accepted to continue in Part B, 37 (60.7%) of whom