Page 100 - Read Online
P. 100
Davidson et al. J Cancer Metastasis Treat 2021;7:45 https://dx.doi.org/10.20517/2394-4722.2021.77 Page 3 of 19
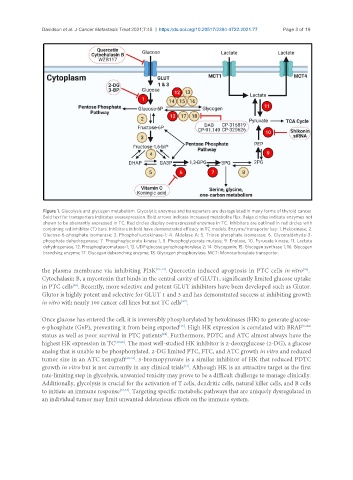
Figure 1. Glycolysis and glycogen metabolism. Glycolytic enzymes and transporters are dysregulated in many forms of thyroid cancer.
Bold text for transporters indicates overexpression. Bold arrows indicate increased metabolite flux. Beige circles indicate enzymes not
shown to be aberrantly expressed in TC. Red circles display overexpressed enzymes in TC. Inhibitors are outlined in red circles with
conjoining red inhibitor (T) bars. Inhibitors in bold have demonstrated efficacy in TC models. Enzyme/transporter key: 1. Hekoxinase; 2.
Glucose-6-phosphate isomerase; 3. Phosphofructokinase-1; 4. Aldolase A; 5. Triose phosphate isomerase; 6. Glyceraldehyde-3-
phosphate dehydrogenase; 7. Phosphoglycerate kinase 1; 8. Phosphoglycerate mutase; 9. Enolase; 10. Pyruvate kinase; 11. Lactate
dehydrogenase, 12. Phosphoglucomutase-1; 13. UDP-glucose pyrophosphorylase 2; 14. Glycogenin; 15. Glycogen synthase 1; 16. Glycogen
branching enzyme; 17. Glycogen debranching enzyme; 18. Glycogen phosphorylase. MCT: Monocarboxylate transporter.
the plasma membrane via inhibiting PI3K [22-24] . Quercetin induced apoptosis in PTC cells in vitro .
[25]
Cytochalasin B, a mycotoxin that binds in the central cavity of GLUT1, significantly limited glucose uptake
[26]
in PTC cells . Recently, more selective and potent GLUT inhibitors have been developed such as Glutor.
Glutor is highly potent and selective for GLUT 1 and 3 and has demonstrated success at inhibiting growth
in vitro with nearly 100 cancer cell lines but not TC cells .
[27]
Once glucose has entered the cell, it is irreversibly phosphorylated by hexokinases (HK) to generate glucose-
6-phosphate (G6P), preventing it from being exported . High HK expression is correlated with BRAF V600E
[15]
status as well as poor survival in PTC patients . Furthermore, PDTC and ATC almost always have the
[28]
highest HK expression in TC [18,28] . The most well-studied HK inhibitor is 2-deoxyglucose (2-DG), a glucose
analog that is unable to be phosphorylated. 2-DG limited PTC, FTC, and ATC growth in vitro and reduced
tumor size in an ATC xenograft [29-31] . 3-bromopyruvate is a similar inhibitor of HK that reduced PDTC
[32]
growth in vitro but is not currently in any clinical trials . Although HK is an attractive target as the first
rate-limiting step in glycolysis, unwanted toxicity may prove to be a difficult challenge to manage clinically.
Additionally, glycolysis is crucial for the activation of T cells, dendritic cells, natural killer cells, and B cells
to initiate an immune response [33,34] . Targeting specific metabolic pathways that are uniquely dysregulated in
an individual tumor may limit unwanted deleterious effects on the immune system.