Page 74 - Read Online
P. 74
Page 12 of 19 Maurizi et al. J Cancer Metastasis Treat 2021;7:35 https://dx.doi.org/10.20517/2394-4722.2021.74
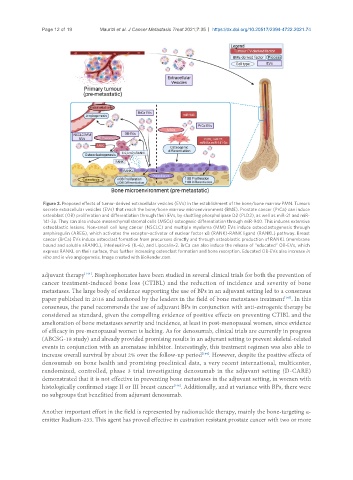
Figure 2. Proposed effects of tumor-derived extracellular vesicles (EVs) in the establishment of the bone/bone marrow PMN. Tumors
secrete extracellular vesicles (EVs) that reach the bone/bone marrow microenvironment (BME). Prostate cancer (PrCa) can induce
osteoblast (OB) proliferation and differentiation through their EVs, by shuttling phospholipase D2 (PLD2), as well as miR-21 and miR-
141-3p. They can also induce mesenchymal stromal cells (MSCs) osteogenic differentiation through miR-940. This induces extensive
osteoblastic lesions. Non-small cell lung cancer (NSCLC) and multiple myeloma (MM) EVs induce osteoclastogenesis through
amphiregulin (AREG), which activates the receptor-activator of nuclear factor κB (RANK)-RANK ligand (RANKL) pathway. Breast
cancer (BrCa) EVs induce osteoclast formation from precursors directly and through osteoblastic production of RANKL (membrane
bound and soluble sRANKL), interleukin-6 (IL-6), and Lipocalin-2. BrCa can also induce the release of “educated” OB-EVs, which
express RANKL on their surface, thus further increasing osteoclast formation and bone resorption. Educated OB-EVs also increase in
vitro and in vivo angiogenesis. Image created with BioRender.com.
adjuvant therapy . Bisphosphonates have been studied in several clinical trials for both the prevention of
[144]
cancer treatment-induced bone loss (CTIBL) and the reduction of incidence and severity of bone
metastases. The large body of evidence supporting the use of BPs in an adjuvant setting led to a consensus
paper published in 2016 and authored by the leaders in the field of bone metastases treatment . In this
[145]
consensus, the panel recommends the use of adjuvant BPs in conjunction with anti-estrogenic therapy be
considered as standard, given the compelling evidence of positive effects on preventing CTIBL and the
amelioration of bone metastases severity and incidence, at least in post-menopausal women, since evidence
of efficacy in pre-menopausal women is lacking. As for denosumab, clinical trials are currently in progress
(ABCSG-18 study) and already provided promising results in an adjuvant setting to prevent skeletal-related
events in conjunction with an aromatase inhibitor. Interestingly, this treatment regimen was also able to
[144]
increase overall survival by about 2% over the follow-up period . However, despite the positive effects of
denosumab on bone health and promising preclinical data, a very recent international, multicenter,
randomized, controlled, phase 3 trial investigating denosumab in the adjuvant setting (D-CARE)
demonstrated that it is not effective in preventing bone metastases in the adjuvant setting, in women with
histologically confirmed stage II or III breast cancer . Additionally, and at variance with BPs, there were
[146]
no subgroups that benefitted from adjuvant denosumab.
Another important effort in the field is represented by radionuclide therapy, mainly the bone-targeting α-
emitter Radium-233. This agent has proved effective in castration resistant prostate cancer with two or more