Page 91 - Read Online
P. 91
Goyal et al. J Cancer Metastasis Treat 2021;7:18 https://dx.doi.org/10.20517/2394-4722.2020.143 Page 7 of 12
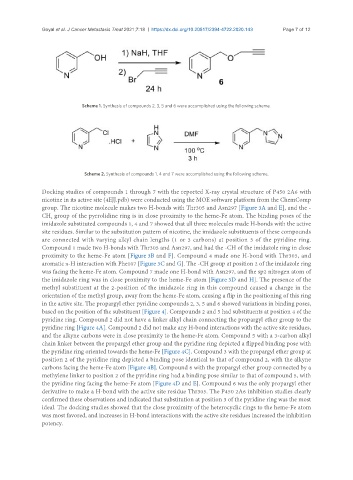
Scheme 1. Synthesis of compounds 2, 3, 5 and 6 were accomplished using the following scheme.
Scheme 2. Synthesis of compounds 1, 4 and 7 were accomplished using the following scheme.
Docking studies of compounds 1 through 7 with the reported X-ray crystal structure of P450 2A6 with
nicotine in its active site (4EJJ.pdb) were conducted using the MOE software platform from the ChemComp
group. The nicotine molecule makes two H-bonds with Thr305 and Asn297 [Figure 3A and E], and the -
CH group of the pyrrolidine ring is in close proximity to the heme-Fe atom. The binding poses of the
2
imidazole substituted compounds 1, 4 and 7 showed that all three molecules made H-bonds with the active
site residues. Similar to the substitution pattern of nicotine, the imidazole substituents of these compounds
are connected with varying alkyl chain lengths (1 or 3 carbons) at position 3 of the pyridine ring.
Compound 1 made two H-bonds with Thr305 and Asn297, and had the -CH of the imidazole ring in close
proximity to the heme-Fe atom [Figure 3B and F]. Compound 4 made one H-bond with Thr305, and
aromatic π-H interaction with Phe107 [Figure 3C and G]. The -CH group at position 2 of the imidazole ring
was facing the heme-Fe atom. Compound 7 made one H-bond with Asn297, and the sp2 nitrogen atom of
the imidazole ring was in close proximity to the heme-Fe atom [Figure 3D and H]. The presence of the
methyl substituent at the 2-position of the imidazole ring in this compound caused a change in the
orientation of the methyl group, away from the heme-Fe atom, causing a flip in the positioning of this ring
in the active site. The propargyl ether pyridine compounds 2, 3, 5 and 6 showed variations in binding poses,
based on the position of the substituent [Figure 4]. Compounds 2 and 5 had substituents at position 4 of the
pyridine ring. Compound 2 did not have a linker alkyl chain connecting the propargyl ether group to the
pyridine ring [Figure 4A]. Compound 2 did not make any H-bond interactions with the active site residues,
and the alkyne carbons were in close proximity to the heme-Fe atom. Compound 5 with a 3-carbon alkyl
chain linker between the propargyl ether group and the pyridine ring depicted a flipped binding pose with
the pyridine ring oriented towards the heme-Fe [Figure 4C]. Compound 3 with the propargyl ether group at
position 2 of the pyridine ring depicted a binding pose identical to that of compound 2, with the alkyne
carbons facing the heme-Fe atom [Figure 4B]. Compound 6 with the propargyl ether group connected by a
methylene linker to position 2 of the pyridine ring had a binding pose similar to that of compound 5, with
the pyridine ring facing the heme-Fe atom [Figure 4D and E]. Compound 6 was the only propargyl ether
derivative to make a H-bond with the active site residue Thr305. The P450 2A6 inhibition studies clearly
confirmed these observations and indicated that substitution at position 3 of the pyridine ring was the most
ideal. The docking studies showed that the close proximity of the heterocyclic rings to the heme-Fe atom
was most favored, and increases in H-bond interactions with the active site residues increased the inhibition
potency.