Page 46 - Read Online
P. 46
Page 8 of 15 Girotti et al. J Cancer Metastasis Treat 2020;6:52 I http://dx.doi.org/10.20517/2394-4722.2020.107
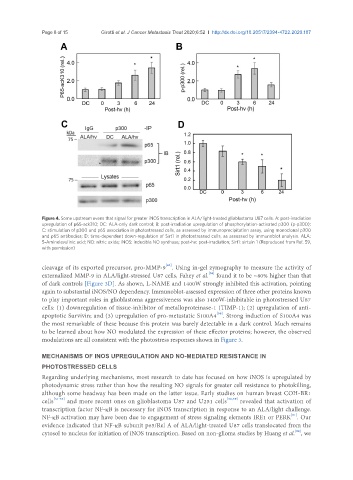
Figure 4. Some upstream evens that signal for greater iNOS transcription in ALA/light-treated glioblastoma U87 cells. A: post-irradiation
upregulation of p65-ack310; DC: ALA-only dark control; B: post-irradiation upregulation of phosphorylation-activated p300 (p-p300);
C: stimulation of p300 and p65 association in photostressed cells, as assessed by immunoprecipitation assay, using monoclonal p300
and p65 antibodies; D: time-dependent down-regulation of Sirt1 in photostressed cells, as assessed by immunoblot analysis. ALA:
5-Aminolevulinic acid; NO: nitric oxide; iNOS: inducible NO synthase; post-hν: post-irradiation; Sirt1: sirtuin 1 (Reproduced from Ref. 59,
with permission)
[60]
cleavage of its exported precursor, pro-MMP-9 . Using in-gel zymography to measure the activity of
[56]
externalized MMP-9 in ALA/light-stressed U87 cells, Fahey et al. found it to be ~80% higher than that
of dark controls [Figure 3D]. As shown, L-NAME and 1400W strongly inhibited this activation, pointing
again to substantial iNOS/NO dependency. Immunoblot-assessed expression of three other proteins known
to play important roles in glioblastoma aggressiveness was also 1400W-inhibitable in photostressed U87
cells: (1) downregulation of tissue-inhibitor of metalloproteinase-1 (TIMP-1); (2) upregulation of anti-
[56]
apoptotic Survivin; and (3) upregulation of pro-metastatic S100A4 . Strong induction of S100A4 was
the most remarkable of these because this protein was barely detectable in a dark control. Much remains
to be learned about how NO modulated the expression of these effector proteins; however, the observed
modulations are all consistent with the photostress responses shown in Figure 3.
MECHANISMS OF INOS UPREGULATION AND NO-MEDIATED RESISTANCE IN
PHOTOSTRESSED CELLS
Regarding underlying mechanisms, most research to date has focused on how iNOS is upregulated by
photodynamic stress rather than how the resulting NO signals for greater cell resistance to photokilling,
although some headway has been made on the latter issue. Early studies on human breast COH-BR1
cells [51-53] and more recent ones on glioblastoma U87 and U251 cells [56,59] revealed that activation of
transcription factor NF-κB is necessary for iNOS transcription in response to an ALA/light challenge.
[61]
NF-κB activation may have been due to engagement of stress signaling elements IRE1 or PERK . Our
evidence indicated that NF-κB subunit p65/Rel A of ALA/light-treated U87 cells translocated from the
[62]
cytosol to nucleus for initiation of iNOS transcription. Based on non-glioma studies by Huang et al. , we