Page 44 - Read Online
P. 44
Page 6 of 15 Girotti et al. J Cancer Metastasis Treat 2020;6:52 I http://dx.doi.org/10.20517/2394-4722.2020.107
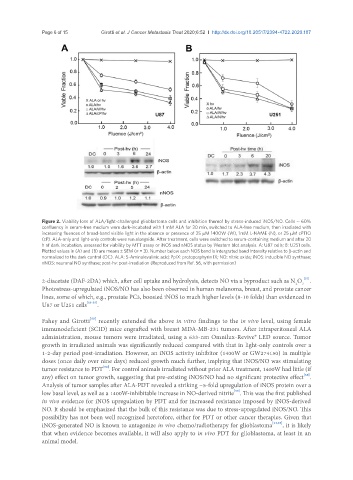
Figure 2. Viability loss of ALA/light-challenged glioblastoma cells and inhibition thereof by stress-induced iNOS/NO. Cells ~ 60%
confluency in serum-free medium were dark-incubated with 1 mM ALA for 30 min, switched to ALA-free medium, then irradiated with
increasing fluences of broad-band visible light in the absence or presence of 25 μM 1400W (W), 1mM L-NAME (N), or 25 μM cPTIO
(cP). ALA-only and light-only controls were run alongside. After treatment, cells were switched to serum-containing medium and after 20
h of dark incubation, assessed for viability by MTT assay or iNOS and nNOS status by Western blot analysis. A: U87 cells; B: U251 cells.
Plotted values in (A) and (B) are means ± SEM (n = 3). Number below each NOS band is intergrated band Intensity relative to β-actin and
normalized to the dark control (DC). ALA: 5-Aminolevulinic acid; PpIX: protoporphyrin IX; NO: nitric oxide; iNOS: inducible NO synthase;
nNOS: neuronal NO synthase; post-hv: post-irradiation (Reproduced from Ref. 56, with permission)
2-diacetate (DAF-2DA) which, after cell uptake and hydrolysis, detects NO via a byproduct such as N O 3 [57] .
2
Photostress-upregulated iNOS/NO has also been observed in human melanoma, breast, and prostate cancer
lines, some of which, e.g., prostate PC3, boosted iNOS to much higher levels (8-10 folds) than evidenced in
U87 or U251 cells [53-55] .
[58]
Fahey and Girotti recently extended the above in vitro findings to the in vivo level, using female
immunodeficient (SCID) mice engrafted with breast MDA-MB-231 tumors. After intraperitoneal ALA
administration, mouse tumors were irradiated, using a 633-nm Omnilux-Revive® LED source. Tumor
growth in irradiated animals was significantly reduced compared with that in light-only controls over a
1-2-day period post-irradiation. However, an iNOS activity inhibitor (1400W or GW274150) in multiple
doses (once daily over nine days) reduced growth much further, implying that iNOS/NO was stimulating
tumor resistance to PDT . For control animals irradiated without prior ALA treatment, 1400W had little (if
[58]
[58]
any) effect on tumor growth, suggesting that pre-existing iNOS/NO had no significant protective effect .
Analysis of tumor samples after ALA-PDT revealed a striking ~5-fold upregulation of iNOS protein over a
[58]
low basal level, as well as a 1400W-inhibitable increase in NO-derived nitrite . This was the first published
in vivo evidence for iNOS upregulation by PDT and for increased resistance imposed by iNOS-derived
NO. It should be emphasized that the bulk of this resistance was due to stress-upregulated iNOS/NO. This
possibility has not been well recognized heretofore, either for PDT or other cancer therapies. Given that
iNOS-generated NO is known to antagonize in vivo chemo/radiotherapy for glioblastoma [24,25] , it is likely
that when evidence becomes available, it will also apply to in vivo PDT for glioblastoma, at least in an
animal model.