Page 29 - Read Online
P. 29
Pellerino et al. J Cancer Metastasis Treat 2020;6:41 I http://dx.doi.org/10.20517/2394-4722.2020.80 Page 11 of 20
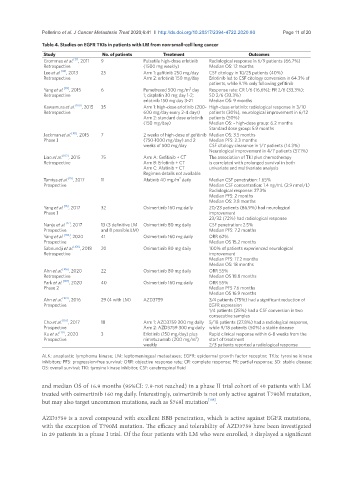
Table 4. Studies on EGFR TKIs in patients with LM from non-small-cell lung cancer
Study No. of patients Treatment Outcomes
Grommes et al. [31] , 2011 9 Pulsatile high-dose erlotinib Radiological response in 6/9 patients (66.7%)
Retrospective (1500 mg weekly) Median OS: 12 months
Lee et al. [98] , 2013 25 Arm 1: gefitinib 250 mg/day CSF citology in 10/25 patients (40%)
Retrospective Arm 2: erlotinib 150 mg/day Erlotinib led to CSF citology conversion in 64.3% of
patients, while 9.1% only following gefitinib
2
Yang et al. [99] , 2015 6 Pemetrexed 500 mg/m day Response rate: CR 1/6 (16.6%); PR 2/6 (33.3%);
Retrospective 1; cisplatin 30 mg day 1-2; SD 2/6 (33.3%)
erlotinib 150 mg day 3-21 Median OS: 9 months
Kawamura et al. [100] , 2015 35 Arm 1: high-dose erlotinib (200- High-dose erlotinib: radiological response in 3/10
Retrospective 600 mg/day every 2-4 days) patients (30%), neurological improvement in 6/12
Arm 2: standard dose erlotinib patients (50%)
(150 mg/day) Median OS: - high-dose group: 6.2 months
Standard dose group: 5.9 months
Jackman et al. [101] , 2015 7 2 weeks of high-dose of gefitinib Median OS: 3.5 months
Phase I (750-1000 mg/day) and 2 Median PFS: 2.3 months
weeks of 500 mg/day CSF citology clearance in 1/7 patients (14.3%)
Neurological improvement in 4/7 patients (57.1%)
Liao et al. [102] , 2015 75 Arm A: Gefitinib + CT The association of TKI plus chemotherapy
Retrospective Arm B: Erlotinib + CT is correlated with prolonged survival in both
Arm C: Afatinib + CT univariate and multivariate analysis
Regimen details not available
2
Tamiya et al. [15] , 2017 11 Afatinib 40 mg/m daily Median CSF penetration: 1.65%
Prospective Median CSF concentration: 1.4 ng/mL (2.9 nmol/L)
Radiological response: 27.3%
Median PFS: 2 months
Median OS: 3.8 months
Yang et al. [16] , 2017 32 Osimertinib 160 mg daily 20/23 patients (86.9%) had neurological
Phase I improvement
23/32 (72%) had radiological response
Nanjo et al. [17] , 2017 13 (3 definitive LM Osimertinib 80 mg daily CSF penetration: 2.5%
Prospective and 8 possible LM) Median PFS: 7.2 months
Yang et al. [104] , 2020 41 Osimertinib 160 mg daily ORR 62%
Prospective Median OS 15.2 months
Saboundji et al. [105] , 2018 20 Osimertinib 80 mg daily 100% of patients experienced neurological
Retrospective improvement
Median PFS: 17.2 months
Median OS: 18 months
Ahn et al. [106] , 2020 22 Osimertinib 80 mg daily ORR 55%
Retrospective Median OS 18.8 months
Park et al. [107] , 2020 40 Osimertinib 160 mg daily ORR 55%
Phase 2 Median PFS 7.6 months
Median OS 16.9 months
Ahn et al. [109] , 2016 29 (4 with LM) AZD3759 3/4 patients (75%) had a significant reduction of
Prospective EGFR expression
1/4 patients (25%) had a CSF conversion in two
consecutive samples
Cho et al. [110] , 2017 18 Arm 1: AZD3759 200 mg daily 5/18 patients (27.8%) had a radiological response,
Prospective Arm 2: AZD3759 300 mg daily while 9/18 patients (50%) a stable disease
Xu et al. [112] , 2020 3 Erlotinib (150 mg/day) plus Rapid clinical response within 6-8 weeks from the
2
Prospective nimotuzumab (200 mg/m ) start of treatment
weekly 2/3 patients reported a radiological response
ALK: anaplastic lymphoma kinase; LM: leptomeningeal metastases; EGFR: epidermal growth factor receptor; TKIs: tyrosine kinase
inhibitors; PFS: progression-free survival; ORR: objective response rate; CR: complete response; PR: partial response; SD: stable disease;
OS: overall survival; TKI: tyrosine kinase inhibitor; CSF: cerebrospinal fluid
and median OS of 16.9 months (95%CI: 7.9-not reached) in a phase II trial cohort of 40 patients with LM
treated with osimertinib 160 mg daily. Interestingly, osimertinib is not only active against T790M mutation,
[108]
but may also target uncommon mutations, such as S768I mutation .
AZD3759 is a novel compound with excellent BBB penetration, which is active against EGFR mutations,
with the exception of T790M mutation. The efficacy and tolerability of AZD3759 have been investigated
in 29 patients in a phase I trial. Of the four patients with LM who were enrolled, 3 displayed a significant