Page 9 - Read Online
P. 9
Page 4 of 21 Su et al. J Cancer Metastasis Treat 2020;6:19 I http://dx.doi.org/10.20517/2394-4722.2020.48
A
B
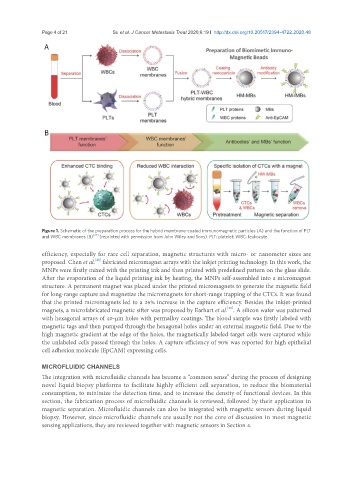
Figure 1. Schematic of the preparation process for the hybrid membrane-coated immunomagnetic particles (A) and the function of PLT
and WBC membranes (B) [27] (reprinted with permission from John Wiley and Sons). PLT: platelet; WBC: leukocyte
efficiency, especially for rare cell separation, magnetic structures with micro- or nanometer sizes are
proposed. Chen et al. fabricated micromagnet arrays with the inkjet printing technology. In this work, the
[25]
MNPs were firstly mixed with the printing ink and then printed with predefined pattern on the glass slide.
After the evaporation of the liquid printing ink by heating, the MNPs self-assembled into a micromagnet
structure. A permanent magnet was placed under the printed micromagnets to generate the magnetic field
for long-range capture and magnetize the micromagnets for short-range trapping of the CTCs. It was found
that the printed micromagnets led to a 26% increase in the capture efficiency. Besides the inkjet-printed
magnets, a microfabricated magnetic sifter was proposed by Earhart et al. . A silicon wafer was patterned
[28]
with hexagonal arrays of 40-μm holes with permalloy coatings. The blood sample was firstly labeled with
magnetic tags and then pumped through the hexagonal holes under an external magnetic field. Due to the
high magnetic gradient at the edge of the holes, the magnetically labeled target cells were captured while
the unlabeled cells passed through the holes. A capture efficiency of 90% was reported for high epithelial
cell adhesion molecule (EpCAM) expressing cells.
MICROFLUIDIC CHANNELS
The integration with microfluidic channels has become a “common sense” during the process of designing
novel liquid biopsy platforms to facilitate highly efficient cell separation, to reduce the biomaterial
consumption, to minimize the detection time, and to increase the density of functional devices. In this
section, the fabrication process of microfluidic channels is reviewed, followed by their application in
magnetic separation. Microfluidic channels can also be integrated with magnetic sensors during liquid
biopsy. However, since microfluidic channels are usually not the core of discussion in most magnetic
sensing applications, they are reviewed together with magnetic sensors in Section 4.