Page 11 - Read Online
P. 11
Page 6 of 21 Su et al. J Cancer Metastasis Treat 2020;6:19 I http://dx.doi.org/10.20517/2394-4722.2020.48
A
B
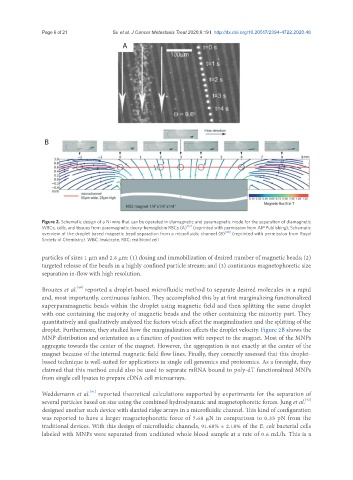
Figure 2. Schematic design of a Ni wire that can be operated in diamagnetic and paramagnetic mode for the separation of diamagnetic
WBCs, cells, and tissues from paramagnetic deoxy-hemoglobin RBCs (A) [37] (reprinted with permission from AIP Publishing); Schematic
overview of the droplet based magnetic bead separation from a microfluidic channel (B) [40] (reprinted with permission from Royal
Society of Chemistry). WBC: leukocyte; RBC: red blood cell
particles of sizes 1 μm and 2.8 μm: (1) dosing and immobilization of desired number of magnetic beads; (2)
targeted release of the beads in a highly confined particle stream; and (3) continuous magnetophoretic size
separation in-flow with high resolution.
[40]
Brouzes et al. reported a droplet-based microfluidic method to separate desired molecules in a rapid
and, most importantly, continuous fashion. They accomplished this by at first marginalizing functionalized
superparamagnetic beads within the droplet using magnetic field and then splitting the same droplet
with one containing the majority of magnetic beads and the other containing the minority part. They
quantitatively and qualitatively analyzed the factors which affect the marginalization and the splitting of the
droplet. Furthermore, they studied how the marginalization affects the droplet velocity. Figure 2B shows the
MNP distribution and orientation as a function of position with respect to the magnet. Most of the MNPs
aggregate towards the center of the magnet. However, the aggregation is not exactly at the center of the
magnet because of the internal magnetic field flow lines. Finally, they correctly assessed that this droplet-
based technique is well-suited for applications in single cell genomics and proteomics. As a foresight, they
claimed that this method could also be used to separate mRNA bound to poly-dT functionalized MNPs
from single cell lysates to prepare cDNA cell microarrays.
[41]
Weddemann et al. reported theoretical calculations supported by experiments for the separation of
[42]
several particles based on size using the combined hydrodynamic and magnetophoretic forces. Jung et al.
designed another such device with slanted ridge arrays in a microfluidic channel. This kind of configuration
was reported to have a larger magnetophoretic force of 7.68 μN in comparison to 0.35 pN from the
traditional devices. With this design of microfluidic channels, 91.68% ± 2.18% of the E. coli bacterial cells
labeled with MNPs were separated from undiluted whole blood sample at a rate of 0.6 mL/h. This is a