Page 57 - Read Online
P. 57
Webster et al. J Cancer Metastasis Treat 2020;6:8 I http://dx.doi.org/10.20517/2394-4722.2019.38 Page 5 of 14
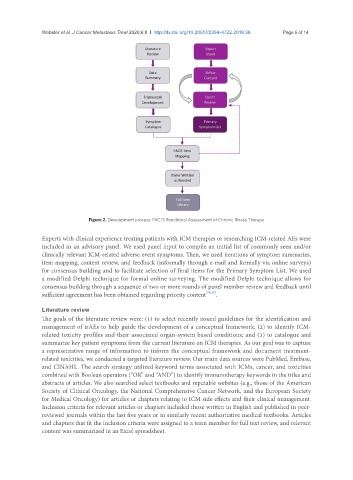
Figure 2. Development process. FACIT: Functional Assessment of Chronic Illness Therapy
Experts with clinical experience treating patients with ICM therapies or researching ICM-related AEs were
included in an advisory panel. We used panel input to compile an initial list of commonly seen and/or
clinically relevant ICM-related adverse event symptoms. Then, we used iterations of symptom summaries,
item mapping, content review, and feedback (informally through e-mail and formally via online surveys)
for consensus building and to facilitate selection of final items for the Primary Symptom List. We used
a modified Delphi technique for formal online surveying. The modified Delphi technique allows for
consensus building through a sequence of two or more rounds of panel member review and feedback until
sufficient agreement has been obtained regarding priority content [34,35] .
Literature review
The goals of the literature review were: (1) to select recently issued guidelines for the identification and
management of irAEs to help guide the development of a conceptual framework; (2) to identify ICM-
related toxicity profiles and their associated organ-system based conditions; and (3) to catalogue and
summarize key patient symptoms from the current literature on ICM therapies. As our goal was to capture
a representative range of information to inform the conceptual framework and document treatment-
related toxicities, we conducted a targeted literature review. Our main data sources were PubMed, Embase,
and CINAHL. The search strategy utilized keyword terms associated with ICMs, cancer, and toxicities
combined with Boolean operators (“OR” and “AND”) to identify immunotherapy keywords in the titles and
abstracts of articles. We also searched select textbooks and reputable websites (e.g., those of the American
Society of Clinical Oncology, the National Comprehensive Cancer Network, and the European Society
for Medical Oncology) for articles or chapters relating to ICM side effects and their clinical management.
Inclusion criteria for relevant articles or chapters included those written in English and published in peer-
reviewed journals within the last five years or in similarly recent authoritative medical textbooks. Articles
and chapters that fit the inclusion criteria were assigned to a team member for full text review, and relevant
content was summarized in an Excel spreadsheet.