Page 21 - Read Online
P. 21
Page 6 of 11 Tulotta et al. J Cancer Metastasis Treat 2019;5:74 I http://dx.doi.org/10.20517/2394-4722.2019.022
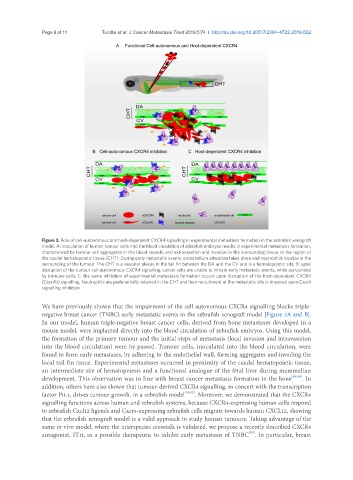
Figure 3. Role of cell-autonomous and host-dependent CXCR4 signalling in experimental metastasis formation in the zebrafish xenograft
model. A: inoculation of human tumour cells into the blood circulation of zebrafish embryos results in experimental metastasis formation,
characterized by tumour cell aggregates in the blood vessels, and extravasation and invasion in the surrounding tissue, in the region of
the caudal hematopoietic tissue (CHT). During early metastatic events, endothelium alteration takes place and neutrophils localize in the
surrounding of the tumour. The CHT is a vascular plexus in the tail fin between the DA and the CV and is a hematopoietic site; B: upon
disruption of the tumour cell-autonomous CXCR4 signalling, cancer cells are unable to initiate early metastatic events, while surrounded
by immune cells; C: the same inhibition of experimental metastasis formation occurs upon disruption of the host-dependent CXCR4
(Cxcr4b) signalling. Neutrophils are preferentially retained in the CHT and their recruitment at the metastatic site is impaired upon Cxcr4
signalling inhibition
We have previously shown that the impairment of the cell autonomous CXCR4 signalling blocks triple-
negative breast cancer (TNBC) early metastatic events in the zebrafish xenograft model [Figure 3A and B].
In our model, human triple-negative breast cancer cells, derived from bone metastases developed in a
mouse model, were implanted directly into the blood circulation of zebrafish embryos. Using this model,
the formation of the primary tumour and the initial steps of metastasis (local invasion and intravasation
into the blood circulation) were by-passed. Tumour cells, inoculated into the blood circulation, were
found to form early metastases, by adhering to the endothelial wall, forming aggregates and invading the
local tail fin tissue. Experimental metastases occurred in proximity of the caudal hematopoietic tissue,
an intermediate site of hematopoiesis and a functional analogue of the fetal liver during mammalian
development. This observation was in line with breast cancer metastasis formation in the bone [89,90] . In
addition, others have also shown that tumour-derived CXCR4 signalling, in concert with the transcription
factor Pit-1, drives tumour growth, in a zebrafish model [91,92] . Moreover, we demonstrated that the CXCR4
signalling functions across human and zebrafish systems, because CXCR4-expressing human cells respond
to zebrafish Cxcl12 ligands and Cxcr4-expressing zebrafish cells migrate towards human CXCL12, showing
that the zebrafish xenograft model is a valid approach to study human tumours. Taking advantage of the
same in vivo model, where the interspecies crosstalk is validated, we propose a recently described CXCR4
[93]
antagonist, IT1t, as a possible therapeutic to inhibit early metastasis of TNBC . In particular, breast