Page 326 - Read Online
P. 326
Flynn et al. J Cancer Metastasis Treat 2019;5:43 I http://dx.doi.org/10.20517/2394-4722.2019.13 Page 3 of 12
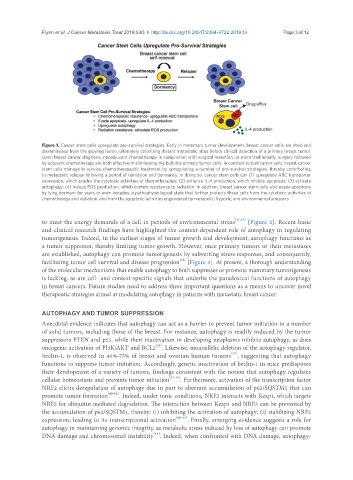
Figure 1. Cancer stem cells upregulate pro-survival strategies. Early in mammary tumor development, breast cancer cells are shed and
disseminated from the growing lesion, ultimately colonizing distant metastatic sites before clinical detection of a primary breast tumor.
Upon breast cancer diagnosis, neoadjuvant chemotherapy in conjunction with surgical resection, or more traditionally, surgery followed
by adjuvant chemotherapy are both effective in eliminating the bulk the primary tumor cells. In contrast to bulk tumor cells, breast cancer
stem cells manage to survive chemotherapeutic treatment by upregulating a number of pro-survival strategies, thereby contributing
to metastatic relapse following a period of remission and dormancy. In doing so, cancer stem cells can (1) upregulate ABC transporter
expression, which evades the cytotoxic activities of chemotherapies; (2) enhance IL-4 production, which inhibits apoptosis; (3) activate
autophagy; (4) induce ROS production, which confers resistance to radiation. In addition, breast cancer stem cells also evade apoptosis
by lying dormant for years or even decades, a pathophysiological state that further protects these cells from the cytotoxic activities of
chemotherapy and radiation, and from the apoptotic activities engendered by metabolic, hypoxic, and environmental stressors
to meet the energy demands of a cell in periods of environmental stress [32,34] [Figure 2]. Recent basic
and clinical research findings have highlighted the context-dependent role of autophagy in regulating
tumorigenesis. Indeed, in the earliest stages of tumor growth and development, autophagy functions as
a tumor suppressor, thereby limiting tumor growth. However, once primary tumors or their metastases
are established, autophagy can promote tumorigenesis by subverting stress responses, and consequently,
[35]
facilitating tumor cell survival and disease progression [Figure 3]. At present, a thorough understanding
of the molecular mechanisms that enable autophagy to both suppresses or promote mammary tumorigenesis
is lacking, as are cell- and context-specific signals that underlie the paradoxical functions of autophagy
in breast cancers. Future studies need to address these important questions as a means to uncover novel
therapeutic strategies aimed at modulating autophagy in patients with metastatic breast cancer.
AUTOPHAGY AND TUMOR SUPPRESSION
Anecdotal evidence indicates that autophagy can act as a barrier to prevent tumor initiation in a number
of solid tumors, including those of the breast. For instance, autophagy is readily induced by the tumor
suppressors PTEN and p53, while their inactivation in developing neoplasms inhibits autophagy, as does
[36]
oncogenic activation of PI3K/AKT and BCL2 . Likewise, monoallelic deletion of the autophagy regulator,
[37]
beclin-1, is observed in 40%-75% of breast and ovarian human tumors , suggesting that autophagy
functions to suppress tumor initiation. Accordingly, genetic inactivation of beclin-1 in mice predisposes
their development of a variety of tumors, findings consistent with the notion that autophagy regulates
cellular homeostasis and prevents tumor initiation [37-39] . Furthermore, activation of the transcription factor
NRF2 elicits deregulation of autophagy due in part to aberrant accumulation of p62/SQSTM1 that can
promote tumor formation [40-42] . Indeed, under tonic conditions, NRF2 interacts with Keap1, which targets
NRF2 for ubiquitin-mediated degradation. The interaction between Keap1 and NRF2 can be prevented by
the accumulation of p62/SQSTM1, thereby: (1) inhibiting the activation of autophagy; (2) stabilizing NRF2
expression, leading to its transcriptional activation [40-43] . Finally, emerging evidence suggests a role for
autophagy in maintaining genomic integrity, as metabolic stress induced by loss of autophagy can promote
[44]
DNA damage and chromosomal instability . Indeed, when confronted with DNA damage, autophagy-