Page 331 - Read Online
P. 331
Page 8 of 12 Flynn et al. J Cancer Metastasis Treat 2019;5:43 I http://dx.doi.org/10.20517/2394-4722.2019.13
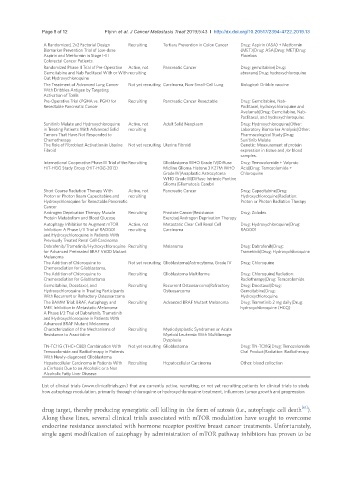
A Randomized, 2x2 Factorial Design Recruiting Tertiary Prevention in Colon Cancer Drug: Aspirin (ASA) + Metformin
Biomarker Prevention Trial of Low-dose (MET)|Drug: ASA|Drug: MET|Drug:
Aspirin and Metformin in Stage I-III Placebos
Colorectal Cancer Patients.
Randomized Phase II Trial of Pre-Operative Active, not Pancreatic Cancer Drug: gemcitabine|Drug:
Gemcitabine and Nab Paclitacel With or With recruiting abraxane|Drug: hydroxychloroquine
Out Hydroxychloroquine
The Treatment of Advanced Lung Cancer Not yet recruiting Carcinoma, Non-Small-Cell Lung Biological: Dribble vaccine
With Dribbles Antigen by Targeting
Activation of Tcells
Pre-Operative Trial (PGHA vs. PGH) for Recruiting Pancreatic Cancer Resectable Drug: Gemcitabine, Nab-
Resectable Pancreatic Cancer Paclitaxel, hydroxychloroquine and
Avelumab|Drug: Gemcitabine, Nab-
Paclitaxel, and hydroxychloroquine
Sunitinib Malate and Hydroxychloroquine Active, not Adult Solid Neoplasm Drug: Hydroxychloroquine|Other:
in Treating Patients With Advanced Solid recruiting Laboratory Biomarker Analysis|Other:
Tumors That Have Not Responded to Pharmacological Study|Drug:
Chemotherapy Sunitinib Malate
The Role of Fibroblast Activation in Uterine Not yet recruiting Uterine Fibroid Genetic: Measurement of protein
Fibroid expression in tissue and /or blood
samples.
International Cooperative Phase III Trial of the Recruiting Glioblastoma WHO Grade IV|Diffuse Drug: Temozolomide + Valproic
HIT-HGG Study Group (HIT-HGG-2013) Midline Glioma Histone 3 K27M WHO Acid|Drug: Temozolomide +
Grade IV|Anaplastic Astrocytoma Chloroquine
WHO Grade III|Diffuse Intrinsic Pontine
Glioma|Gliomatosis Cerebri
Short Course Radiation Therapy With Active, not Pancreatic Cancer Drug: Capecitabine|Drug:
Proton or Photon Beam Capecitabine and recruiting Hydroxychloroquine|Radiation:
Hydroxychloroquine for Resectable Pancreatic Proton or Photon Radiation Therapy
Cancer
Androgen Deprivation Therapy Muscle Recruiting Prostate Cancer|Resistance Drug: Zoladex
Protein Metabolism and Blood Glucose Exercise|Androgen Deprivation Therapy
Autophagy Inhibition to Augment mTOR Active, not Metastatic Clear Cell Renal Cell Drug: Hydroxychloroquine|Drug:
Inhibition: A Phase I/II Trial of RAD001 recruiting Carcinoma RAD001
and Hydroxychloroquine in Patients With
Previously Treated Renal Cell Carcinoma
Dabrafenib/Trametinib/Hydroxychloroquine Recruiting Melanoma Drug: Dabrafenib|Drug:
for Advanced Pretreated BRAF V600 Mutant Trametinib|Drug: Hydroxychloroquine
Melanoma
The Addition of Chloroquine to Not yet recruiting Glioblastoma|Astrocytoma, Grade IV Drug: Chloroquine
Chemoradiation for Glioblastoma,
The Addition of Chloroquine to Recruiting Glioblastoma Multiforme Drug: Chloroquine|Radiation:
Chemoradiation for Glioblastoma Radiotherapy|Drug: Temozolomide
Gemcitabine, Docetaxel, and Recruiting Recurrent Osteosarcoma|Refractory Drug: Docetaxel|Drug:
Hydroxychloroquine in Treating Participants Osteosarcoma Gemcitabine|Drug:
With Recurrent or Refractory Osteosarcoma Hydroxychloroquine
The BAMM Trial: BRAF, Autophagy and Recruiting Advanced BRAF Mutant Melanoma Drug: Trametinib 2 mg daily|Drug:
MEK Inhibition in Metastatic Melanoma: hydroxychloroquine (HCQ)
A Phase I/2 Trial of Dabrafenib, Trametinib
and Hydroxychloroquine in Patients With
Advanced BRAF Mutant Melanoma
Characterization of the Mechanisms of Recruiting Myelodysplastic Syndromes or Acute
Resistance to Azacitidine Myeloid Leukemia With Multilineage
Dysplasia
TN-TC11G (THC+CBD) Combination With Not yet recruiting Glioblastoma Drug: TN-TC11G|Drug: Temozolomide
Temozolomide and Radiotherapy in Patients Oral Product|Radiation: Radiotherapy
With Newly-diagnosed Glioblastoma
Hepatocellular Carcinoma in Patients With Recruiting Hepatocellular Carcinoma Other: blood collection
a Cirrhosis Due to an Alcoholic or a Non
Alcoholic Fatty Liver Disease
List of clinical trials (www.clinicaltrials.gov) that are currently active, recruiting, or not yet recruiting patients for clinical trials to study
how autophagy modulation, primarily through chloroquine or hydroxychloroquine treatment, influences tumor growth and progression
[83]
drug target, thereby producing synergistic cell killing in the form of autosis (i.e., autophagic cell death ).
Along these lines, several clinical trials associated with mTOR modulation have sought to overcome
endocrine resistance associated with hormone receptor positive breast cancer treatments. Unfortunately,
single agent modification of autophagy by administration of mTOR pathway inhibitors has proven to be