Page 304 - Read Online
P. 304
Page 4 of 9 Robinson et al. J Cancer Metastasis Treat 2019;5:39 I http://dx.doi.org/10.20517/2394-4722.2019.15
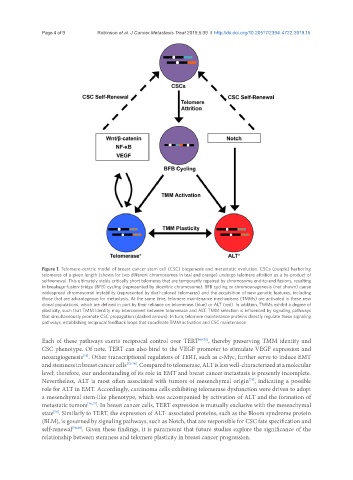
Figure 1. Telomere-centric model of breast cancer stem cell (CSC) biogenesis and metastatic evolution. CSCs (purple) harboring
telomeres of a given length (shown for two different chromosomes in teal and orange) undergo telomere attrition as a by-product of
self-renewal. This ultimately yields critically short telomeres that are temporarily repaired by chromosome end-to-end fusions, resulting
in breakage-fusion-bridge (BFB) cycling (represented by dicentric chromosome). BFB cycling or chromoanagenesis (not shown) cause
widespread chromosomal instability (represented by dual-colored telomeres) and the acquisition of new genetic features, including
those that are advantageous for metastasis. At the same time, telomere maintenance mechanisms (TMMs) are activated in these new
clonal populations, which are defined in part by their reliance on telomerase (blue) or ALT (red). In addition, TMMs exhibit a degree of
plasticity, such that TMM identity may interconvert between telomerase and ALT. TMM selection is influenced by signaling pathways
that simultaneously promote CSC propagation (dashed arrows). In turn, telomere maintenance proteins directly regulate these signaling
pathways, establishing reciprocal feedback loops that coordinate TMM activation and CSC maintenance
Each of these pathways exerts reciprocal control over TERT [69,70] , thereby preserving TMM identity and
CSC phenotype. Of note, TERT can also bind to the VEGF promoter to stimulate VEGF expression and
neoangiogenesis . Other transcriptional regulators of TERT, such as c-Myc, further serve to induce EMT
[71]
and stemness in breast cancer cells [72-74] . Compared to telomerase, ALT is less well-characterized at a molecular
level; therefore, our understanding of its role in EMT and breast cancer metastasis is presently incomplete.
[75]
Nevertheless, ALT is most often associated with tumors of mesenchymal origin , indicating a possible
role for ALT in EMT. Accordingly, carcinoma cells exhibiting telomerase dysfunction were driven to adopt
a mesenchymal stem-like phenotype, which was accompanied by activation of ALT and the formation of
metastatic tumors [76,77] . In breast cancer cells, TERT expression is mutually exclusive with the mesenchymal
[78]
state . Similarly to TERT, the expression of ALT- associated proteins, such as the Bloom syndrome protein
(BLM), is governed by signaling pathways, such as Notch, that are responsible for CSC fate specification and
self-renewal [79,80] . Given these findings, it is paramount that future studies explore the significance of the
relationship between stemness and telomere plasticity in breast cancer progression.