Page 264 - Read Online
P. 264
Page 6 of 11 Mizejewski. J Cancer Metastasis Treat 2019;5:35 I http://dx.doi.org/10.20517/2394-4722.2018.70
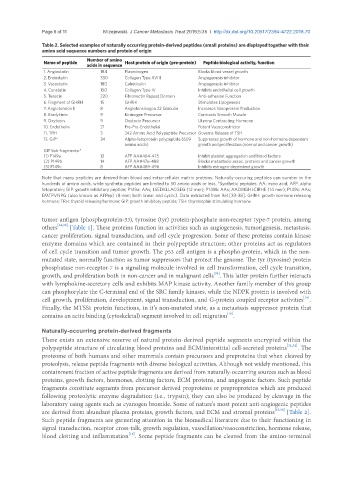
Table 2. Selected examples of naturally occurring protein-derived peptides (small proteins) are displayed together with their
amino acid sequence numbers and protein of origin
Name of peptide Number of amino Host protein of origin (pre-protein) Peptide biological activity, function
acids in sequence
1. Angiostatin 184 Plasminogen Blocks blood vessel growth
2. Endostatin 330 Collagen Type XVIII Angiogenesis inhibitor
3. Vasostatin 180 Calreticulin Angiogenesis inhibitor
4. Constatin 150 Collagen Type IV Inhibits endothelial cell growth
5. Tenacin 220 Fibronectin Repeat Domain Anti-adhesion Function
6. Fragment of GHRH 15 GHRH Stimulates Lipogenesis
7. Angiotension II 8 Angiotensinogen 22 Globulin Increases Vasopressin Production
8. Bradykinin 9 Kininogen Precursor Contracts Smooth Muscle
9. Oxytocin 9 Oxytocin Precursor Uterine Contracting Hormone
10. Endothelin 21 Pre-Pro-Endothelial Potent Vasoconstrictor
11. TRH 3 242 Amino Acid Polypeptide Precursor Governs Release of TSH
12. GIP* 34 Alpha-fetoprotein polypeptide (609 Suppresses growth of hormone and non-hormone dependent
amino acids) growth and proliferation (normal and cancer growth)
GIP Sub-fragments:*
(1) P149a 12 AFP AA#464-475 Inhibit platelet aggregation and blood factors
(2) P149b 14 AFP AA#476-488 Blocks metastasis-assoc. proteins and cancer growth
(3) P149c 8 AFP AA#489-496 Inhibits estrogen-dependent growth
Note that many peptides are derived from blood and extra-cellular matrix proteins. Naturally-occuring peptides can number in the
hundreds of amino acids, while synthetic peptides are limited to 50 amino acids or less. *Synthetic peptides. AA: mino acid; AFP: alpha
fetoprotein; GIP: growth inhibitory peptide; P149a: AAs; LSEDKLLACGEG (12 mer); P149b: AAs; AADIIIGHLCIRHE (14 mer); P149c: AAs;
EMTPVNPG (also known as AFPep) (8-mer; both linear and cyclic). Data extracted from Ref.[33-38]. GHRH: growth hormone releasing
hormone; TRH: thyroid releasing hormone; GIP: growth inhibitory peptide; TSH: thyrotrophin stimulating hormone
tumor antigen (phosphoprotein-53), tyrosine (tyr) protein-phosphate non-receptor type-7 protein, among
others [28,29] [Table 1]. These proteins function in activities such as angiogenesis, tumorigenesis, metastasis,
cancer proliferation, signal transduction, and cell cycle progression. Some of these proteins contain kinase
enzyme domains which are contained in their polypeptide structure; other proteins act as regulators
of cell cycle transition and tumor growth. The p53 cell antigen is a phospho-protein, which in the non-
mutated state, normally function as tumor suppressors that protect the genome. The tyr (tyrosine) protein
phosphatase non-receptor-7 is a signaling molecule involved in cell transformation, cell cycle transition,
[30]
growth, and proliferation both in non-cancer and in malignant cells . This latter protein further interacts
with lymphokine-secretory cells and exhibits MAP kinase activity. Another family member of this group
can phosphorylate the C-terminal end of the SRC family kinases, while the NDPK protein is involved with
[16]
cell growth, proliferation, development, signal transduction, and G-protein coupled receptor activities .
Finally, the MTSS1 protein functions, in it’s non-mutated state, as a metastasis suppressor protein that
[16]
contains an actin binding (cytoskeletal) segment involved in cell migration .
Naturally-occurring protein-derived fragments
There exists an extensive reserve of natural protein-derived peptide segments encrypted within the
polypeptide structure of circulating blood proteins and ECM/interstitial cell-secreted proteins [31,32] . The
proteome of both humans and other mammals contain precursors and preproteins that when cleaved by
proteolysis, release peptide fragments with diverse biological activities. Although not widely mentioned, this
containment fraction of active peptide fragments are derived from naturally-occurring sources such as blood
proteins, growth factors, hormones, clotting factors, ECM proteins, and angiogenic factors. Such peptide
fragments constitute segments from precursor derived proproteins or preproproteins which are produced
following proteolytic enzyme degradation (i.e., trypsin); they can also be produced by cleavage in the
laboratory using agents such as cyanogen bromide. Some of nature’s most potent anti-angiogenic peptides
are derived from abundant plasma proteins, growth factors, and ECM and stromal proteins [33,36] [Table 2].
Such peptide fragments are garnering attention in the biomedical literature due to their functioning in
signal transduction, receptor cross-talk, growth regulation, vasodilation/vasoconstriction, hormone release,
[16]
blood clotting and inflammation . Some peptide fragments can be cleaved from the amino-terminal