Page 9 - Read Online
P. 9
Page 4 of 8 Wartena et al. J Cancer Metastasis Treat 2018;4:59 I http://dx.doi.org/10.20517/2394-4722.2018.66
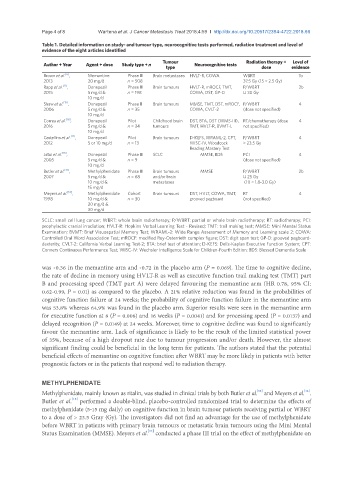
Table 1. Detailed information on study- and tumour type, neurocognitive tests performed, radiation treatment and level of
evidence of the eight articles identified
Tumour Radiation therapy + Level of
Author + Year Agent + dose Study type + n Neurocognitive tests
type dose evidence
[6]
Brown et al. , Memantine Phase III Brain metastases HVLT-R, COWA WBRT 1b
2013 20 mg/d n = 508 37.5 Gy (15 × 2.5 Gy)
[11]
Rapp et al. , Donepezil Phase III Brain tumours HVLT-R, mROCF, TMT, P/WBRT 2b
2015 5 mg/d & n = 198 COWA, DST, GP-D ≥ 30 Gy
10 mg/d
[15]
Shaw et al. , Donepezil Phase II Brain tumours MMSE, TMT, DST, mROCF, P/WBRT 4
2006 5 mg/d & n = 35 COWA, CVLT-2 (dose not specified)
10 mg/d
Correa et al. [16] , Donepezil Pilot Childhood brain DST, BTA, DST (WMS-III), RT/chemotherapy (dose 4
2016 5 mg/d & n = 24 tumours TMT, HVLT-R, BVMT-L not specified)
10 mg/d
[17]
Castellino et al. , Donepezil Pilot Brain tumours D-KEFS, WRAML-2, CPT, P/WBRT 4
2012 5 or 10 mg/d n = 13 WISC-IV, Woodcock > 23.5 Gy
Reading Mastery Test
Jatoi et al. [18] , Donepezil Phase III SCLC MMSE, BDS PCI 4
2005 5 mg/d & n = 9 (dose not specified)
10 mg/d
[13]
Butler et al. , Methylphenidate Phase III Brain tumours MMSE P/WBRT 2b
2007 5 mg/d & n = 68 and/or brain ≥ 25 Gy
10 mg/d & metastases (10 × 1.8-3.0 Gy)
15 mg/d
Meyers et al. [14] , Methylphenidate Cohort Brain tumours DST, HVLT, COWA, TMT, RT 4
1998 10 mg/d & n = 30 grooved pegboard (not specified)
20 mg/d &
30 mg/d
SCLC: small cell lung cancer; WBRT: whole brain radiotherapy; P/WBRT: partial or whole brain radiotherapy; RT: radiotherapy; PCI:
prophylactic cranial irradiation; HVLT-R: Hopkins Verbal Learning Test - Revised; TMT: trail making test; MMSE: Mini Mental Status
Examination; BVMT: Brief Visuospatial Memory Test; WRAML-2: Wide Range Assessment of Memory and Learning scale 2; COWA:
Controlled Oral Word Association Test; mROCF: modified Rey-Osterrieth complex figure; DST: digit span test; GP-D: grooved pegboard-
dexterity; CVLT-2: California Verbal Learning Test-2; BTA: brief test of attention; D-KEFS: Delis-Kaplan Executive Function System; CPT:
Conners Continuous Performance Test; WISC-IV: Wechsler Intelligence Scale for Children-Fourth Edition; BDS: Blessed Dementia Scale
was -0.36 in the memantine arm and -0.72 in the placebo arm (P = 0.069). The time to cognitive decline,
the rate of decline in memory using HVLT-R as well as executive function trail making test (TMT) part
B and processing speed (TMT part A) were delayed favouring the memantine arm (HR 0.78, 95% CI:
0.62-0.99, P = 0.01) as compared to the placebo. A 21% relative reduction was found in the probabilities of
cognitive function failure at 24 weeks; the probability of cognitive function failure in the memantine arm
was 53.8% whereas 64.9% was found in the placebo arm. Superior results were seen in the memantine arm
for executive function at 8 (P = 0.008) and 16 weeks (P = 0.0041) and for processing speed (P = 0.0137) and
delayed recognition (P = 0.0149) at 24 weeks. Moreover, time to cognitive decline was found to significantly
favour the memantine arm. Lack of significance is likely to be the result of the limited statistical power
of 35%, because of a high dropout rate due to tumour progression and/or death. However, the almost
significant finding could be beneficial in the long term for patients. The authors stated that the potential
beneficial effects of memantine on cognitive function after WBRT may be more likely in patients with better
prognostic factors or in the patients that respond well to radiation therapy.
METHYLPHENIDATE
[14]
[13]
Methylphenidate, mainly known as ritalin, was studied in clinical trials by both Butler et al. and Meyers et al. .
Butler et al. performed a double-blind, placebo-controlled randomized trial to determine the effects of
[13]
methylphenidate (5-15 mg daily) on cognitive function in brain tumour patients receiving partial or WBRT
to a dose of > 23.5 Gray (Gy). The investigators did not find an advantage for the use of methylphenidate
before WBRT in patients with primary brain tumours or metastatic brain tumours using the Mini Mental
[14]
Status Examination (MMSE). Meyers et al. conducted a phase III trial on the effect of methylphenidate on