Page 38 - Read Online
P. 38
Page 4 of 10 Dumane et al. J Cancer Metastasis Treat 2019;5:42 I http://dx.doi.org/10.20517/2394-4722.2019.08
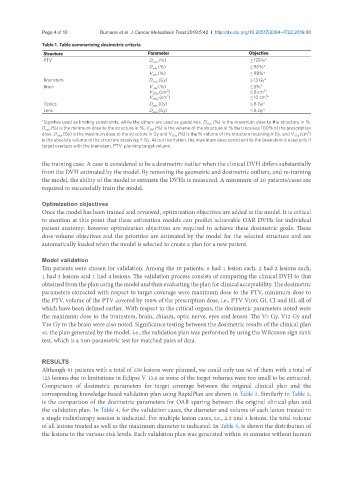
Table 1. Table summarizing dosimetric criteria
Structure Parameter Objective
PTV D max (%) ≤ 125%*
D min (%) ≥ 90%*
V 100 (%) ≥ 98%*
Brainstem D max (Gy) ≤ 13 Gy*
Brain V 7Gy (%) ≤ 5%*
3
3
V 12Gy (cm ) ≤ 8 cm *
3
3
V 10Gy (cm ) ≤ 10 cm *
Optics D max (Gy) ≤ 8 Gy*
Lens D max (Gy) ≤ 8 Gy*
*Signifies used as limiting constraints, while the others are used as guidelines. D max (%) is the maximum dose to the structure in %,
D min (%) is the minimum dose to the structure in %, V 100 (%) is the volume of the structure in % that receives 100% of the prescription
3
dose. D max (Gy) is the maximum dose to the structure in Gy and V XGy (%) is the % volume of the structure receiving X Gy, and V YGy (cm )
is the absolute volume of the structure receiving Y Gy. At our institution, the maximum dose constraint to the brainstem is used only if
target overlaps with the brainstem. PTV: planning target volume
the training case. A case is considered to be a dosimetric outlier when the clinical DVH differs substantially
from the DVH estimated by the model. By removing the geometric and dosimetric outliers, and re-training
the model, the ability of the model to estimate the DVHs is measured. A minimum of 20 patients/cases are
required to successfully train the model.
Optimization objectives
Once the model has been trained and reviewed, optimization objectives are added to the model. It is critical
to mention at this point that these estimation models can predict achievable OAR DVHs for individual
patient anatomy; however optimization objectives are required to achieve these dosimetric goals. These
dose-volume objectives and the priorities are estimated by the model for the selected structure and are
automatically loaded when the model is selected to create a plan for a new patient.
Model validation
Ten patients were chosen for validation. Among the 10 patients, 6 had 1 lesion each, 2 had 2 lesions each,
1 had 3 lesions and 1 had 4 lesions. The validation process consists of comparing the clinical DVH to that
obtained from the plan using the model and then evaluating the plan for clinical acceptability. The dosimetric
parameters extracted with respect to target coverage were maximum dose to the PTV, minimum dose to
the PTV, volume of the PTV covered by 100% of the prescription dose, i.e., PTV V100, GI, CI and HI, all of
which have been defined earlier. With respect to the critical organs, the dosimetric parameters noted were
the maximum dose to the brainstem, brain, chiasm, optic nerve, eyes and lenses. The V7 Gy, V12 Gy and
V10 Gy to the brain were also noted. Significance testing between the dosimetric results of the clinical plan
vs. the plan generated by the model, i.e., the validation plan was performed by using the Wilcoxon sign rank
test, which is a non-parametric test for matched pairs of data.
RESULTS
Although 91 patients with a total of 139 lesions were planned, we could only use 66 of them with a total of
125 lesions due to limitations in Eclipse V 13.6 as some of the target volumes were too small to be extracted.
Comparison of dosimetric parameters for target coverage between the original clinical plan and the
corresponding knowledge-based validation plan using RapidPlan are shown in Table 2. Similarly in Table 3,
is the comparison of the dosimetric parameters for OAR sparing between the original clinical plan and
the validation plan. In Table 4, for the validation cases, the diameter and volume of each lesion treated in
a single radiotherapy session is indicated. For multiple lesion cases, i.e., 2.3 and 4 lesions, the total volume
of all lesions treated as well as the maximum diameter is indicated. In Table 5, is shown the distribution of
the lesions to the various risk levels. Each validation plan was generated within 30 minutes without human