Page 65 - Read Online
P. 65
Page 6 of 13 Eng et al. J Cancer Metastasis Treat 2019;5:69 I http://dx.doi.org/10.20517/2394-4722.2019.021
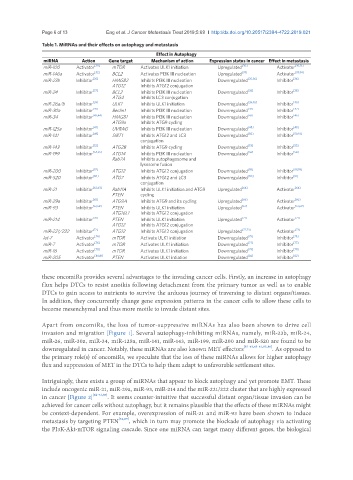
Table 1. MiRNAs and their effects on autophagy and metastasis
Effect in Autophagy
miRNA Action Gene target Mechanism of action Expression status in cancer Effect in metastasis
miR-100 Activator [30] mTOR Activates ULK1 initiation Upregulated [30] Activator [30,31]
miR-146a Activator [32] BCL2 Activates PI3K III nucleation Upregulated [33] Activator [33,34]
miR-23b Inhibitor [35] HMGB2 Inhibits PI3K III nucleation Downregulated [35,36] Inhibitor [36]
ATG12 Inhibits ATG12 conjugation
miR-24 Inhibitor [37] BCL2 Inhibits PI3K III nucleation Downregulated [38] Inhibitor [38]
ATG4 Inhibits LC3 conjugation
miR-26a/b Inhibitor [39] ULK1 Inhibits ULK1 initiation Downregulated [39,40] Inhibitor [40]
miR-30a Inhibitor [41] Beclin1 Inhibits PI3K III nucleation Downregulated [42] Inhibitor [42]
miR-34 Inhibitor [43,44] HMGB1 Inhibits PI3K III nucleation Downregulated [45] Inhibitor [46]
ATG9a Inhibits ATG9 cycling
miR-125a Inhibitor [47] UVRAG Inhibits PI3K III nucleation Downregulated [48] Inhibitor [48]
miR-141 Inhibitor [49] SIRT1 Inhibits ATG12 and LC3 Downregulated [50] Inhibitor [50,51]
conjugation
miR-143 Inhibitor [52] ATG2B Inhibits ATG9 cycling Downregulated [53] Inhibitor [53]
miR-199 Inhibitor [54,55] ATG14 Inhibits PI3K III nucleation Downregulated [56] Inhibitor [56]
Rab7A Inhibits autophagosome and
lysosome fusion
miR-200 Inhibitor [57] ATG12 Inhibits ATG12 conjugation Downregulated [58] Inhibitor [58,59]
miR-520 Inhibitor [60] ATG7 Inhibits ATG12 and LC3 Downregulated [60] Inhibitor [61]
conjugation
miR-21 Inhibitor [62,63] Rab11A Inhibits ULK1 initiation and ATG9 Upregulated [64] Activator [64]
PTEN cycling
miR-29a Inhibitor [65] ATG9A Inhibits ATG9 and its cycling Upregulated [66] Activator [66]
miR-93 Inhibitor [67,68] PTEN Inhibits ULK1 initiation Upregulated [67] Activator [67,69]
ATG16L1 Inhibits ATG12 conjugation
miR-214 Inhibitor [70] PTEN Inhibits ULK1 initiation Upregulated [71] Activator [71]
ATG12 Inhibits ATG12 conjugation
miR-221/222 Inhibitor [72] ATG12 Inhibits ATG12 conjugation Upregulated [72,73] Activator [73]
let-7 Activator [74] mTOR Activate ULK1 initiation Downregulated [75] Inhibitor [75]
miR-7 Activator [76] mTOR Activates ULK1 initiation Downregulated [77] Inhibitor [77]
miR-16 Activator [78] mTOR Activates ULK1 initiation Downregulated [79] Inhibitor [79]
miR-205 Activator [80,81] PTEN Activates ULK1 intiation Downregulated [82] Inhibitor [82]
these oncomiRs provides several advantages to the invading cancer cells. Firstly, an increase in autophagy
flux helps DTCs to resist anoikis following detachment from the primary tumor as well as to enable
DTCs to gain access to nutrients to survive the arduous journey of traversing to distant organs/tissues.
In addition, they concurrently change gene expression patterns in the cancer cells to allow these cells to
become mesenchymal and thus more motile to invade distant sites.
Apart from oncomiRs, the loss of tumor-suppressive miRNAs has also been shown to drive cell
invasion and migration [Figure 1]. Several autophagy-inhibiting miRNAs, namely, miR-23b, miR-24,
miR-26, miR-30a, miR-34, miR-125a, miR-141, miR-143, miR-199, miR-200 and miR-520 are found to be
downregulated in cancer. Notably, these miRNAs are also known MET effectors [35-43,45-61,85,86] . As opposed to
the primary role(s) of oncomiRs, we speculate that the loss of these miRNAs allows for higher autophagy
flux and suppression of MET in the DTCs to help them adapt to unfavorable settlement sites.
Intriguingly, there exists a group of miRNAs that appear to block autophagy and yet promote EMT. These
include oncogenic miR-21, miR-29a, miR-93, miR-214 and the miR-221/222 cluster that are highly expressed
in cancer [Figure 2] [62-73,87] . It seems counter-intuitive that successful distant organ/tissue invasion can be
achieved for cancer cells without autophagy, but it remains plausible that the effects of these miRNAs might
be context-dependent. For example, overexpression of miR-21 and miR-93 have been shown to induce
metastasis by targeting PTEN [64,69] , which in turn may promote the blockade of autophagy via activating
the PI3K-Akt-mTOR signaling cascade. Since one miRNA can target many different genes, the biological