Page 35 - Read Online
P. 35
Kondapuram et al. J Cancer Metastasis Treat 2019;5:32 I http://dx.doi.org/10.20517/2394-4722.2018.105 Page 13 of 25
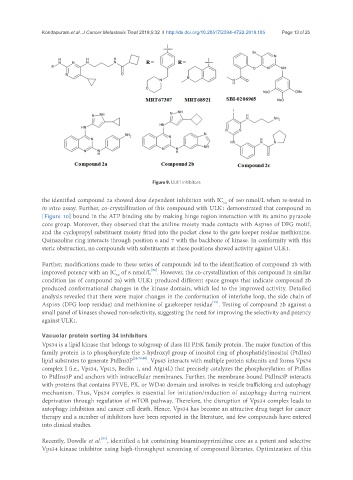
Figure 9. ULK1 inhibitors
the identified compound 2a showed dose dependent inhibition with IC of 160 nmol/L when re-tested in
50
in vitro assay. Further, co-crystallization of this compound with ULK1 demonstrated that compound 2a
[Figure 10] bound in the ATP binding site by making hinge region interaction with its amino pyrazole
core group. Moreover, they observed that the aniline moiety made contacts with Asp165 of DFG motif,
and the cyclopropyl substituent moiety fitted into the pocket close to the gate keeper residue methionine.
Quinazoline ring interacts through position 6 and 7 with the backbone of kinase. In conformity with this
steric obstruction, no compounds with substituents at these positions showed activity against ULK1.
Further, modifications made to these series of compounds led to the identification of compound 2b with
[56]
improved potency with an IC of 8 nmol/L . However, the co-crystallization of this compound in similar
50
condition (as of compound 2a) with ULK1 produced different space groups that indicate compound 2b
produced conformational changes in the kinase domain, which led to the improved activity. Detailed
analysis revealed that there were major changes in the conformation of interlobe loop, the side chain of
[75]
Asp165 (DFG loop residue) and methionine of gatekeeper residue . Testing of compound 2b against a
small panel of kinases showed non-selectivity, suggesting the need for improving the selectivity and potency
against ULK1.
Vacuolar protein sorting 34 inhibitors
Vps34 is a lipid kinase that belongs to subgroup of class III PI3K family protein. The major function of this
family protein is to phosphorylate the 3-hydroxyl group of inositol ring of phosphatidylinositol (PtdIns)
lipid substrates to generate PtdIns3P [22,79,80] . Vps43 interacts with multiple protein subunits and forms Vps34
complex I (i.e., Vps34, Vps15, Beclin 1, and Atg14L) that precisely catalyzes the phosphorylation of PtdIns
to PtdIns3P and anchors with intracellular membranes. Further, the membrane-bound PtdIns3P interacts
with proteins that contains FYVE, PX, or WD40 domain and involves in vesicle trafficking and autophagy
mechanism. Thus, Vps34 complex is essential for initiation/induction of autophagy during nutrient
deprivation through regulation of mTOR pathway. Therefore, the disruption of Vps34 complex leads to
autophagy inhibition and cancer cell death. Hence, Vps34 has become an attractive drug target for cancer
therapy and a number of inhibitors have been reported in the literature, and few compounds have entered
into clinical studies.
[81]
Recently, Dowdle et al. , identified a hit containing bisaminopyrimidine core as a potent and selective
Vps34 kinase inhibitor using high-throughput screening of compound libraries. Optimization of this