Page 52 - Read Online
P. 52
Jusino et al. J Cancer Metastasis Treat 2018;4:43 I http://dx.doi.org/10.20517/2394-4722.2018.24 Page 5 of 20
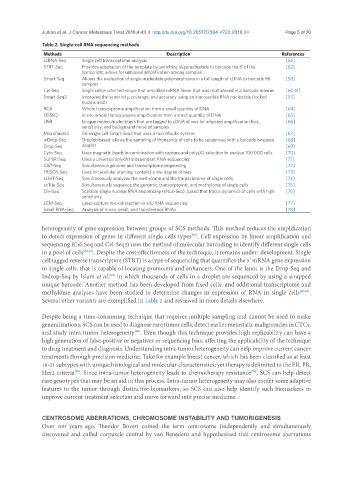
Table 2. Single-cell RNA sequencing methods
Methods Description References
scRNA-Seq Single cell transcriptome analysis [56]
STRT-Seq Provides adaptation of the template by switching oligonucleotide to barcode the 5’ of the [62]
transcripts; allows for unbiased amplification among samples
Smart-Seq Allows the evaluation of single nucleotide polymorphisms in a full length of cDNA to barcode 96 [58]
samples
Cel-Seq Single cell in vitro technique that amplified mRNA linear that was multiplexed in a barcode manner [60,61]
Smart-Seq2 Improved the sensitivity, coverage, and accuracy using an inaccessible RNA nucleotide (locked [57]
nucleic acid)
RCA Whole transcriptome amplification from a small quantity of DNA [64]
FISSEQ In situ whole transcriptome amplification from a small quantity of DNA [65]
UMI Unique molecule identifiers that are tagged to cDNA allows for adjusted amplification bias, [66]
sensitivity, and background noise of samples
Microfluidics 96-single cell Smart-Seq2 that uses a microfluidic system [67]
inDrop-Seq Droplet-based; allows the sampling of thousands of cells to be sequenced with a barcode wrapped [68]
Drop-Seq droplet [69]
Cyto-Seq Uses magnetic beads in combination with capture and poly(A) selection to analyze 100,000 cells [70]
SUPeR-Seq Uses a universal poly(A) independent RNA sequencing [71]
G&T-Seq Simultaneous genome and transcriptome sequencing [72]
FRISCR-Seq Uses intracellular staining; contains a low degree of bias [73]
scMT-Seq Simultaneously analyzes the methylome and the transcriptome of single cells [74]
scTrio-Seq Simultaneously sequence the genomic, transcriptomic, and methylome of single cells [75]
Div-Seq Scalable single nucleus RNA sequencing (sNuc-Seq), based that tracks dynamics of cells with high [76]
sensitivity
LCM-Seq Laser capture microdissection in situ RNA sequencing [77]
Small RNA-Seq Analysis of micro, small, and transference RNAs [78]
heterogeneity of gene expression between groups of SCS methods. This method reduces the amplification
to detect expression of genes in different single cells types . Cell expression by linear amplification and
[59]
sequencing (Cel-Seq and Cel-Seq2) uses the method of molecular barcoding to identify different single cells
in a pool of cells [60,61] . Despite the cost-effectiveness of the technique, it remains under- development. Single
cell tagged reverse transcription (STRT) is a type of sequencing that quantifies the 5’ mRNA gene expression
in single cells, that is capable of locating promoters and enhancers. One of the latest is the Drop-Seq and
Indrop-Seq by Islam et al. in which thousands of cells in a droplet are sequenced by using a wrapped
[62]
unique barcode. Another method has been developed from fixed cells, and additional transcriptome and
methylome analyses have been studied to determine changes in expression of RNA in single cells [47,63] .
Several other variants are exemplified in Table 2 and reviewed in more details elsewhere.
Despite being a time-consuming technique that requires multiple sampling and cannot be used to make
generalizations, SCS can be used to diagnose rare tumor cells, detect earlier metastatic malignancies in CTCs,
and study intra-tumor heterogeneity . Even though this technique provides high replicability can have a
[50]
high generation of false-positive or negatives or sequencing bias, affecting the applicability of the technique
to drug treatment and diagnosis. Understanding intra-tumor heterogeneity can help improve current cancer
treatments through precision medicine. Take for example breast cancer, which has been classified as at least
18-21 subtypes with unique histological and molecular characteristics; yet therapy is delimited to the ER, PR,
Her2 criteria . Since intra-tumor heterogeneity leads to chemotherapy resistance , SCS can help detect
[79]
[79]
rare genotypes that may be an aid in this process. Intra-tumor heterogeneity may also confer some adaptive
features to the tumor through distinctive biomarkers, so SCS can also help identify such biomarkers to
improve current treatment selection and move forward into precise medicine.
CENTROSOME ABERRATIONS, CHROMOSOME INSTABILITY AND TUMORIGENESIS
Over 100 years ago, Theodor Boveri coined the term centrosome (independently and simultaneously
discovered and called corpuscle central by van Beneden) and hypothesized that centrosome aberrations